Multi-level regulation of cellular glycosylation: from genes to transcript to enzyme to structure
- PMID: 27744149
- PMCID: PMC5161581
- DOI: 10.1016/j.sbi.2016.09.013
Multi-level regulation of cellular glycosylation: from genes to transcript to enzyme to structure
Abstract
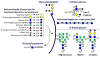
Glycosylation is a ubiquitous mammalian post-translational modification that both decorates a majority of expressed proteins and regulates their function. Cellular glycan biosynthesis is facilitated by a few hundred enzymes that are collectively termed 'glycoenzymes'. The expression and activity of these enzymes is controlled at the transcription, translation and post-translation levels. New wet-lab advances are providing analytical methods to collect large-scale data at these multiple levels, relational databases are starting to collate these results, and computer models are beginning to integrate this information across scales in order to gain new knowledge. These activities are likely to enable the qualitative and quantitative mapping of pathways regulating glycan production and function in proteins, cells and tissue.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

References
-
- Cummings RD. The repertoire of glycan determinants in the human glycome. Mol Biosyst. 2009;5(10):1087–1104. - PubMed
-
- Agrawal P, Kurcon T, Pilobello KT, Rakus JF, Koppolu S, Liu Z, Batista BS, Eng WS, Hsu KL, Liang Y, Mahal LK. Mapping posttranscriptional regulation of the human glycome uncovers microrna defining the glycocode. Proc Natl Acad Sci U S A. 2014;111(11):4338–4343. Maps the lectin binding profile of the NCI-60 cell lines. Identifies microRNA as a key regulator of cellular glycosylation. - PMC - PubMed
-
- Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, Gupta R, et al. Precision mapping of the human o-galnac glycoproteome through simplecell technology. EMBO J. 2013;32(10):1478–1488. Analyzed the spread of O-glycosylation sites using 12 different human cell types that lack the ability to extend O-GalNAc type carbohydrates (SimpleCells). The study identifies 3000 O-glycosites on 600 glycoproteins. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

