The Effect of Dimethyl Fumarate on Cerebral Gray Matter Atrophy in Multiple Sclerosis
- PMID: 27744504
- PMCID: PMC5130921
- DOI: 10.1007/s40120-016-0054-4
The Effect of Dimethyl Fumarate on Cerebral Gray Matter Atrophy in Multiple Sclerosis
Abstract
Introduction: The objective of this pilot study was to compare cerebral gray matter (GM) atrophy over 1 year in patients starting dimethyl fumarate (DMF) for multiple sclerosis (MS) to that of patients on no disease-modifying treatment (noDMT). DMF is an established therapy for relapsing-remitting (RR) MS.
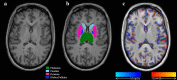
Methods: We retrospectively analyzed 20 patients with RRMS at the start of DMF [age (mean ± SD) 46.1 ± 10.2 years, Expanded Disability Status Scale (EDSS) score 1.1 ± 1.2, timed 25-foot walk (T25FW) 4.6 ± 0.8 s] and eight patients on noDMT (age 42.5 ± 6.6 years, EDSS 1.7 ± 1.1, T25FW 4.4 ± 0.6 s). Baseline and 1-year 3D T1-weighted 3T MRI was processed with automated pipelines (SIENA, FSL-FIRST) to assess percentage whole brain volume change (PBVC) and deep GM (DGM) atrophy. Group differences were assessed by analysis of covariance, with time between MRI scans as a covariate.
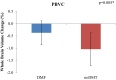
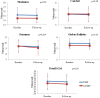
Results: Over 1 year, the DMF group showed a lower rate of whole brain atrophy than the noDMT group (PBVC: -0.37 ± 0.49% vs. -1.04 ± 0.67%, p = 0.005). The DMF group also had less change in putamen volume (-0.06 ± 0.22 vs. -0.32 ± 0.28 ml, p = 0.02). There were no significant on-study differences between groups in caudate, globus pallidus, thalamus, total DGM volume, T2 lesion volume, EDSS, or T25FW (all p > 0.20).
Conclusions: These results suggest a treatment effect of DMF on GM atrophy appearing at 1 year after starting therapy. However, due to the retrospective study design and sample size, these findings should be considered preliminary, and require confirmation in future investigations.
Funding: Biogen.
Keywords: Cerebral gray matter atrophy; Dimethyl fumarate; Multiple sclerosis.
Figures
Similar articles
-
Whole brain and deep gray matter atrophy detection over 5 years with 3T MRI in multiple sclerosis using a variety of automated segmentation pipelines.PLoS One. 2018 Nov 8;13(11):e0206939. doi: 10.1371/journal.pone.0206939. eCollection 2018. PLoS One. 2018. PMID: 30408094 Free PMC article.
-
Automated segmentation of cerebral deep gray matter from MRI scans: effect of field strength on sensitivity and reliability.BMC Neurol. 2017 Sep 5;17(1):172. doi: 10.1186/s12883-017-0949-4. BMC Neurol. 2017. PMID: 28874119 Free PMC article.
-
Sample size requirements for one-year treatment effects using deep gray matter volume from 3T MRI in progressive forms of multiple sclerosis.Int J Neurosci. 2017 Nov;127(11):971-980. doi: 10.1080/00207454.2017.1283313. Epub 2017 Feb 2. Int J Neurosci. 2017. PMID: 28100092
-
Two years' effect of dimethyl fumarate on focal and diffuse gray matter pathology in multiple sclerosis.Mult Scler. 2022 Nov;28(13):2090-2098. doi: 10.1177/13524585221104014. Epub 2022 Jun 28. Mult Scler. 2022. PMID: 35765211
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
Cited by
-
Emerging Understanding of the Mechanism of Action for Dimethyl Fumarate in the Treatment of Multiple Sclerosis.Front Neurol. 2018 Jan 23;9:5. doi: 10.3389/fneur.2018.00005. eCollection 2018. Front Neurol. 2018. PMID: 29410647 Free PMC article. Review.
-
Clinical evaluation of dimethyl fumarate for the treatment of relapsing-remitting multiple sclerosis: efficacy, safety, patient experience and adherence.Patient Prefer Adherence. 2019 Oct 1;13:1655-1666. doi: 10.2147/PPA.S187529. eCollection 2019. Patient Prefer Adherence. 2019. PMID: 31631980 Free PMC article.
-
No Changes in Functional Connectivity After Dimethyl Fumarate Treatment in Multiple Sclerosis.Neurol Ther. 2022 Mar;11(1):471-479. doi: 10.1007/s40120-022-00328-w. Epub 2022 Feb 4. Neurol Ther. 2022. PMID: 35119678 Free PMC article.
-
Gray matter microglial activation in relapsing vs progressive MS: A [F-18]PBR06-PET study.Neurol Neuroimmunol Neuroinflamm. 2019 Jul 1;6(5):e587. doi: 10.1212/NXI.0000000000000587. eCollection 2019 Sep. Neurol Neuroimmunol Neuroinflamm. 2019. PMID: 31355321 Free PMC article.
-
A Narrative Review on Axonal Neuroprotection in Multiple Sclerosis.Neurol Ther. 2022 Sep;11(3):981-1042. doi: 10.1007/s40120-022-00363-7. Epub 2022 May 24. Neurol Ther. 2022. PMID: 35610531 Free PMC article. Review.
References
-
- Filippi M, Wolinsky JS, Comi G. CORAL Study Group. Effects of oral glatiramer acetate on clinical and MRI-monitored disease activity in patients with relapsing multiple sclerosis: a multicentre, double-blind, randomised, placebo-controlled study. Lancet Neurol. 2006;5:213–220. doi: 10.1016/S1474-4422(06)70327-1. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

