Lack of Clinical Pharmacokinetic Studies to Optimize the Treatment of Neglected Tropical Diseases: A Systematic Review
- PMID: 27744580
- PMCID: PMC5425494
- DOI: 10.1007/s40262-016-0467-3
Lack of Clinical Pharmacokinetic Studies to Optimize the Treatment of Neglected Tropical Diseases: A Systematic Review
Abstract
Introduction: Neglected tropical diseases (NTDs) affect more than one billion people, mainly living in developing countries. For most of these NTDs, treatment is suboptimal. To optimize treatment regimens, clinical pharmacokinetic studies are required where they have not been previously conducted to enable the use of pharmacometric modeling and simulation techniques in their application, which can provide substantial advantages.
Objectives: Our aim was to provide a systematic overview and summary of all clinical pharmacokinetic studies in NTDs and to assess the use of pharmacometrics in these studies, as well as to identify which of the NTDs or which treatments have not been sufficiently studied.
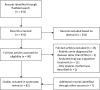
Methods: PubMed was systematically searched for all clinical trials and case reports until the end of 2015 that described the pharmacokinetics of a drug in the context of treating any of the NTDs in patients or healthy volunteers.
Results: Eighty-two pharmacokinetic studies were identified. Most studies included small patient numbers (only five studies included >50 subjects) and only nine (11 %) studies included pediatric patients. A large part of the studies was not very recent; 56 % of studies were published before 2000. Most studies applied non-compartmental analysis methods for pharmacokinetic analysis (62 %). Twelve studies used population-based compartmental analysis (15 %) and eight (10 %) additionally performed simulations or extrapolation. For ten out of the 17 NTDs, none or only very few pharmacokinetic studies could be identified.
Conclusions: For most NTDs, adequate pharmacokinetic studies are lacking and population-based modeling and simulation techniques have not generally been applied. Pharmacokinetic clinical trials that enable population pharmacokinetic modeling are needed to make better use of the available data. Simulation-based studies should be employed to enable the design of improved dosing regimens and more optimally use the limited resources to effectively provide therapy in this neglected area.
Conflict of interest statement
Funding
No specific funding was received for the conduct of this systematic review.
Conflict of interest
TD is a consultant for the non-profit organization Drugs for Neglected Diseases initiative, Geneva, Switzerland.
Figures
References
-
- World Health Organization. Neglected tropical diseases. 2015. http://www.who.int/neglected_diseases/diseases/en/. Accessed 14 Jan 2016.
-
- The END fund; Ending Neglected Diseases. NTD overview. 2015. http://www.end.org/whatwedo/ntdoverview. Accessed 14 Jan 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources