The Landscape of Mouse Meiotic Double-Strand Break Formation, Processing, and Repair
- PMID: 27745971
- PMCID: PMC5117687
- DOI: 10.1016/j.cell.2016.09.035
The Landscape of Mouse Meiotic Double-Strand Break Formation, Processing, and Repair
Abstract
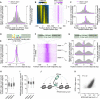
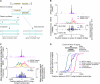
Heritability and genome stability are shaped by meiotic recombination, which is initiated via hundreds of DNA double-strand breaks (DSBs). The distribution of DSBs throughout the genome is not random, but mechanisms molding this landscape remain poorly understood. Here, we exploit genome-wide maps of mouse DSBs at unprecedented nucleotide resolution to uncover previously invisible spatial features of recombination. At fine scale, we reveal a stereotyped hotspot structure-DSBs occur within narrow zones between methylated nucleosomes-and identify relationships between SPO11, chromatin, and the histone methyltransferase PRDM9. At large scale, DSB formation is suppressed on non-homologous portions of the sex chromosomes via the DSB-responsive kinase ATM, which also shapes the autosomal DSB landscape at multiple size scales. We also provide a genome-wide analysis of exonucleolytic DSB resection lengths and elucidate spatial relationships between DSBs and recombination products. Our results paint a comprehensive picture of features governing successive steps in mammalian meiotic recombination.
Keywords: DNA damage; DNA repair; PRDM9; SPO11; chromatin; double-strand break; homologous recombination; meiosis; mouse; resection.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





References
-
- Bailey TL, Gribskov M. Combining evidence using p-values: application to sequence homology searches. Bioinformatics. 1998;14:48–54. - PubMed
-
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. - PubMed
-
- Baudat F, Imai Y, de Massy B. Meiotic recombination in mammals: localization and regulation. Nat Rev Genet. 2013;14:794–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

