Temperature-Robust Neural Function from Activity-Dependent Ion Channel Regulation
- PMID: 27746024
- PMCID: PMC5111818
- DOI: 10.1016/j.cub.2016.08.061
Temperature-Robust Neural Function from Activity-Dependent Ion Channel Regulation
Abstract
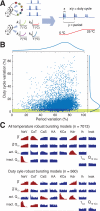
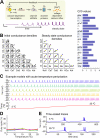
Many species of cold-blooded animals experience substantial and rapid fluctuations in body temperature. Because biological processes are differentially temperature dependent, it is difficult to understand how physiological processes in such animals can be temperature robust [1-8]. Experiments have shown that core neural circuits, such as the pyloric circuit of the crab stomatogastric ganglion (STG), exhibit robust neural activity in spite of large (20°C) temperature fluctuations [3, 5, 7, 8]. This robustness is surprising because (1) each neuron has many different kinds of ion channels with different temperature dependencies (Q10s) that interact in a highly nonlinear way to produce firing patterns and (2) across animals there is substantial variability in conductance densities that nonetheless produce almost identical firing properties. The high variability in conductance densities in these neurons [9, 10] appears to contradict the possibility that robustness is achieved through precise tuning of key temperature-dependent processes. In this paper, we develop a theoretical explanation for how temperature robustness can emerge from a simple regulatory control mechanism that is compatible with highly variable conductance densities [11-13]. The resulting model suggests a general mechanism for how nervous systems and excitable tissues can exploit degenerate relationships among temperature-sensitive processes to achieve robust function.
Keywords: central pattern generator; computational model; crustacean; homeostatic plasticity; ion channels; mathematical model; neuronal excitability; stomatogastric ganglion; temperature compensation.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Homeostasis: How Neurons Achieve Temperature Invariance.Curr Biol. 2016 Nov 7;26(21):R1141-R1143. doi: 10.1016/j.cub.2016.09.063. Curr Biol. 2016. PMID: 27825449
References
-
- Rajan K, Abbott LF. Temperature-compensated chemical reactions. Physical Review E. 2007;75(2):022902. - PubMed
-
- Hong CI, Tyson JJ. A proposal for temperature compensation of the circadian rhythm in Drosophila based on dimerization of the per protein. Chronobiology International. 1997;14:521–529. - PubMed
-
- Robertson RM, Money TG. Temperature and neuronal circuit function: compensation, tuning and tolerance. Curr Opin Neurobiol. 2012;22:724–734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

