Identified Cellular Correlates of Neocortical Ripple and High-Gamma Oscillations during Spindles of Natural Sleep
- PMID: 27746131
- PMCID: PMC5130902
- DOI: 10.1016/j.neuron.2016.09.032
Identified Cellular Correlates of Neocortical Ripple and High-Gamma Oscillations during Spindles of Natural Sleep
Abstract
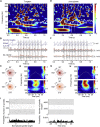
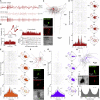
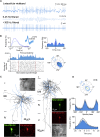
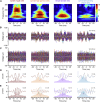
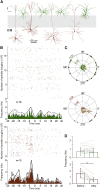
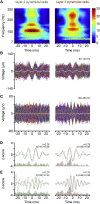
Ultra-high-frequency network events in the hippocampus are instrumental in a dialogue with the neocortex during memory formation, but the existence of transient ∼200 Hz network events in the neocortex is not clear. Our recordings from neocortical layer II/III of freely behaving rats revealed field potential events at ripple and high-gamma frequencies repeatedly occurring at troughs of spindle oscillations during sleep. Juxtacellular recordings identified subpopulations of fast-spiking, parvalbumin-containing basket cells with epochs of firing at ripple (∼200 Hz) and high-gamma (∼120 Hz) frequencies detected during spindles and centered with millisecond precision at the trough of spindle waves in phase with field potential events but phase shifted relative to pyramidal cell firing. The results suggest that basket cell subpopulations are involved in spindle-nested, high-frequency network events that hypothetically provide repeatedly occurring neocortical temporal reference states potentially involved in mnemonic processes.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Bacci A., Huguenard J.R. Enhancement of spike-timing precision by autaptic transmission in neocortical inhibitory interneurons. Neuron. 2006;49:119–130. - PubMed
-
- Beierlein M., Gibson J.R., Connors B.W. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J. Neurophysiol. 2003;90:2987–3000. - PubMed
-
- Born J., Rasch B., Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. - PubMed
-
- Burgalossi A., Herfst L., von Heimendahl M., Förste H., Haskic K., Schmidt M., Brecht M. Microcircuits of functionally identified neurons in the rat medial entorhinal cortex. Neuron. 2011;70:773–786. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

