Optical manipulation of a single human virus for study of viral-cell interactions
- PMID: 27746582
- PMCID: PMC5058341
- DOI: 10.1117/12.2239051
Optical manipulation of a single human virus for study of viral-cell interactions
Abstract
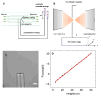
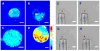
Although Ashkin and Dziedzic first demonstrated optical trapping of individual tobacco mosaic viruses in suspension as early as 1987, this pioneering work has not been followed up only until recently. Using human immunodeficiency virus type 1 (HIV-1) as a model virus, we have recently demonstrated that a single HIV-1 virion can be stabled trapped, manipulated and measured in physiological media with high precision. The capability to optically trap a single virion in suspension not only allows us to determine, for the first time, the refractive index of a single virus with high precision, but also quantitate the heterogeneity among individual virions with single-molecule resolution, the results of which shed light on the molecular mechanisms of virion infectivity. Here we report the further development of a set of microscopic techniques to physically deliver a single HIV-1 virion to a single host cell in solution. Combined with simultaneous epifluorescence imaging, the attachment and dissociation events of individual manipulated virions on host cell surface can be measured and the results help us understand the role of diffusion in mediating viral attachment to host cells. The establishment of these techniques opens up new ways for investigation of a wide range of virion-cell interactions, and should be applicable for study of B cell interactions with particulate antigens such as viruses.
Keywords: HIV-1; Optical trapping; manipulation; micropipette; single cell; single virus; virus-cell interactions.
Figures


References
-
- Ashkin A, Dziedzic J, Bjorkholm J, et al. First demonstration of stable optical trapping of micron-sized dielectric objects in three dimensions using a single-beam gradient optical trap. Opt Lett. 1986;11:288–90. - PubMed
-
- Hou X, Cheng W. Encyclopedia of Biophysics: SpringerReference. 2013. Optical Tweezers; pp. 1800–1807.
-
- MacDonald MP, Spalding GC, Dholakia K. Microfluidic sorting in an optical lattice. Nature. 2003;426(6965):421–424. - PubMed
-
- Ashkin A, Dziedzic JM. Optical trapping and manipulation of viruses and bacteria. Science. 1987;235(4795):1517–20. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
