Vasodilator-stimulated phosphoprotein-phosphorylation by ginsenoside Ro inhibits fibrinogen binding to αIIb/β3 in thrombin-induced human platelets
- PMID: 27746688
- PMCID: PMC5052406
- DOI: 10.1016/j.jgr.2015.11.003
Vasodilator-stimulated phosphoprotein-phosphorylation by ginsenoside Ro inhibits fibrinogen binding to αIIb/β3 in thrombin-induced human platelets
Abstract
Background: Glycoprotein IIb/IIIa (αIIb/β3) is involved in platelet adhesion, and triggers a series of intracellular signaling cascades, leading to platelet shape change, granule secretion, and clot retraction. In this study, we evaluated the effect of ginsenoside Ro (G-Ro) on the binding of fibrinogen to αIIb/β3.
Methods: We investigated the effect of G-Ro on regulation of signaling molecules affecting the binding of fibrinogen to αIIb/β3, and its final reaction, clot retraction.
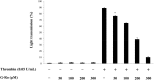
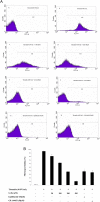
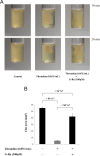
Results: We found that G-Ro dose-dependently inhibited thrombin-induced platelet aggregation and attenuated the binding of fibrinogen to αIIb/β3 by phosphorylating cyclic adenosine monophosphate (cAMP)-dependently vasodilator-stimulated phosphoprotein (VASP; Ser157). In addition, G-Ro strongly abrogated the clot retraction reflecting the intensification of thrombus.
Conclusion: We demonstrate that G-Ro is a beneficial novel compound inhibiting αIIb/β3-mediated fibrinogen binding, and may prevent platelet aggregation-mediated thrombotic disease.
Keywords: VASP (Ser157); cAMP; clot retraction; fibrinogen binding; ginsenoside Ro.
Figures


Similar articles
-
Total saponin from Korean Red Ginseng inhibits binding of adhesive proteins to glycoprotein IIb/IIIa via phosphorylation of VASP (Ser(157)) and dephosphorylation of PI3K and Akt.J Ginseng Res. 2016 Jan;40(1):76-85. doi: 10.1016/j.jgr.2015.05.004. Epub 2015 May 21. J Ginseng Res. 2016. PMID: 26843825 Free PMC article.
-
Inhibitory Effects of Ginsenoside Ro on Clot Retraction through Suppressing PI3K/Akt Signaling Pathway in Human Platelets.Prev Nutr Food Sci. 2019 Mar;24(1):56-63. doi: 10.3746/pnf.2019.24.1.56. Epub 2019 Mar 31. Prev Nutr Food Sci. 2019. PMID: 31008097 Free PMC article.
-
Anti-thrombotic effects of ginsenoside Rk3 by regulating cAMP and PI3K/MAPK pathway on human platelets.J Ginseng Res. 2023 Nov;47(6):706-713. doi: 10.1016/j.jgr.2023.04.006. Epub 2023 Apr 22. J Ginseng Res. 2023. PMID: 38107398 Free PMC article.
-
Platelet glycoprotein IIb/IIIa antagonists: lessons learned from clinical trials and future directions.Crit Care Med. 2002 May;30(5 Suppl):S332-40. doi: 10.1097/00003246-200205001-00025. Crit Care Med. 2002. PMID: 12004256 Review.
-
Recent development of peptides from glycoproteins IIb (alphaIIb) and IIIa (beta3) that inhibit platelet fibrinogen binding.Curr Med Chem Cardiovasc Hematol Agents. 2005 Apr;3(2):99-103. doi: 10.2174/1568016053544345. Curr Med Chem Cardiovasc Hematol Agents. 2005. PMID: 15853697 Review.
Cited by
-
Saponins as Modulators of the Blood Coagulation System and Perspectives Regarding Their Use in the Prevention of Venous Thromboembolic Incidents.Molecules. 2020 Nov 6;25(21):5171. doi: 10.3390/molecules25215171. Molecules. 2020. PMID: 33172028 Free PMC article. Review.
-
Structural characteristics, bioavailability and cardioprotective potential of saponins.Integr Med Res. 2018 Mar;7(1):33-43. doi: 10.1016/j.imr.2018.01.003. Epub 2018 Feb 3. Integr Med Res. 2018. PMID: 29629289 Free PMC article. Review.
-
Ginsenosides in Panax genus and their biosynthesis.Acta Pharm Sin B. 2021 Jul;11(7):1813-1834. doi: 10.1016/j.apsb.2020.12.017. Epub 2021 Jan 2. Acta Pharm Sin B. 2021. PMID: 34386322 Free PMC article. Review.
-
Daidzein Inhibits Human Platelet Activation by Downregulating Thromboxane A2 Production and Granule Release, Regardless of COX-1 Activity.Int J Mol Sci. 2023 Jul 26;24(15):11985. doi: 10.3390/ijms241511985. Int J Mol Sci. 2023. PMID: 37569361 Free PMC article.
-
Inhibitory effects of thromboxane A2 generation by ginsenoside Ro due to attenuation of cytosolic phospholipase A2 phosphorylation and arachidonic acid release.J Ginseng Res. 2019 Apr;43(2):236-241. doi: 10.1016/j.jgr.2017.12.007. Epub 2018 Jan 9. J Ginseng Res. 2019. PMID: 30976161 Free PMC article.
References
-
- Payrastre B., Missy K., Trumel C., Bodin S., Plantavid M., Chap H. The integrin alpha IIb/beta 3 in human platelet signal transduction. Biochem Pharmacol. 2000;60:1069–1074. - PubMed
-
- Phillips D.R., Nannizzi-Alaimo L., Prasad K.S. Beta3 tyrosine phosphorylation in alphaIIbbeta3 (platelet membrane GP IIb-IIIa) outside-in integrin signaling. Thromb Haemost. 2001;86:246–258. - PubMed
-
- Sudo T., Ito H., Kimura Y. Phosphorylation of the vasodilator-stimulated phosphoprotein (VASP) by the anti-platelet drug, cilostazol, in platelets. Platelets. 2003;14:381–390. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

