Somatostatin-Expressing Inhibitory Interneurons in Cortical Circuits
- PMID: 27746722
- PMCID: PMC5040712
- DOI: 10.3389/fncir.2016.00076
Somatostatin-Expressing Inhibitory Interneurons in Cortical Circuits
Abstract
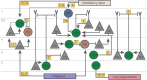
Cortical inhibitory neurons exhibit remarkable diversity in their morphology, connectivity, and synaptic properties. Here, we review the function of somatostatin-expressing (SOM) inhibitory interneurons, focusing largely on sensory cortex. SOM neurons also comprise a number of subpopulations that can be distinguished by their morphology, input and output connectivity, laminar location, firing properties, and expression of molecular markers. Several of these classes of SOM neurons show unique dynamics and characteristics, such as facilitating synapses, specific axonal projections, intralaminar input, and top-down modulation, which suggest possible computational roles. SOM cells can be differentially modulated by behavioral state depending on their class, sensory system, and behavioral paradigm. The functional effects of such modulation have been studied with optogenetic manipulation of SOM cells, which produces effects on learning and memory, task performance, and the integration of cortical activity. Different classes of SOM cells participate in distinct disinhibitory circuits with different inhibitory partners and in different cortical layers. Through these disinhibitory circuits, SOM cells help encode the behavioral relevance of sensory stimuli by regulating the activity of cortical neurons based on subcortical and intracortical modulatory input. Associative learning leads to long-term changes in the strength of connectivity of SOM cells with other neurons, often influencing the strength of inhibitory input they receive. Thus despite their heterogeneity and variability across cortical areas, current evidence shows that SOM neurons perform unique neural computations, forming not only distinct molecular but also functional subclasses of cortical inhibitory interneurons.
Keywords: GIN; Martinotti; VIP; disinhibitory; parvalbumin; somatostatin; x94; x98.
Figures

References
-
- Anderson J. S., Carandini M., Ferster D. (2000). Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J. Neurophysiol. 84, 909–926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

