A Low Spin Manganese(IV) Nitride Single Molecule Magnet
- PMID: 27746891
- PMCID: PMC5058364
- DOI: 10.1039/C6SC01469K
A Low Spin Manganese(IV) Nitride Single Molecule Magnet
Abstract
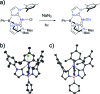
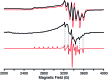
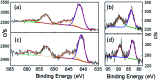
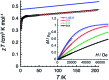
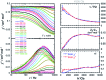
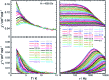
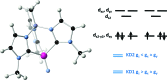
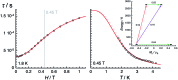
Structural, spectroscopic and magnetic methods have been used to characterize the tris(carbene)borate compound PhB(MesIm)3Mn≡N as a four-coordinate manganese(IV) complex with a low spin (S = 1/2) configuration. The slow relaxation of the magnetization in this complex, i.e. its single-molecule magnet (SMM) properties, is revealed under an applied dc field. Multireference quantum mechanical calculations indicate that this SMM behavior originates from an anisotropic ground doublet stabilized by spin-orbit coupling. Consistent theoretical and experiment data show that the resulting magnetization dynamics in this system is dominated by ground state quantum tunneling, while its temperature dependence is influenced by Raman relaxation.
Figures

References
-
- Freedman D. E., Harman W. H., Harris T. D., Long G. J., Chang C. J., Long J. R. J. Am. Chem. Soc. 2010;132:1224. - PubMed
-
- Abragam A. and Bleaney B., Electron Paramagnetic Resonance of Transition Ions, Dover, New York, 1986.
-
- Gatteschi D., Sessoli R. and Villain J., Molecular Nanomagnets, Oxford University Press, Oxford, 2006.
-
- Zadrozny J. M., Xiao D. J., Atanasov M., Long G. J., Grandjean F., Neese F., Long J. R. Nat. Chem. 2013;5:577. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

