Arousal, motor control, and parkinson's disease
- PMID: 27747095
- PMCID: PMC4936629
- DOI: 10.1515/tnsci-2015-0021
Arousal, motor control, and parkinson's disease
Abstract
This review highlights the most important discovery in the reticular activating system (RAS) in the last 10 years, the manifestation of gamma (γ) band activity in cells of the RAS, especially in the pedunculopontine nucleus (PPN), which is in charge of the high frequency states of waking and rapid eye movement sleep. This discovery is critical to understanding the modulation of movement by the RAS and how it sets the background over which we generate voluntary and triggered movements. The presence of γ band activity in the RAS is proposed to participate in the process of preconscious awareness, and provide the essential stream of information for the formulation of many of our actions. Early findings using stimulation of this region to induce arousal, and also to elicit stepping, are placed in this context. This finding also helps explain the novel use of PPN deep brain stimulation for the treatment of Parkinson's disease, although considerable work remains to be done.
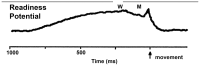
Keywords: Arousal; Calcium channels; Deep brain stimulation; Mu rhythm; P13 potential; P50 potential; Parkinson’s disease; Readiness potential.
Conflict of interest statement
The authors have no conflicts of interest.
Figures

References
-
- Llinas RR. I of the Vortex; from neurons to self. MIT Press; Cambridge, MA, USA: 2001.
-
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, et al. Coherent oscillations: a mechanism of feature linking in the visual system? Biol Cybern. 1988;60:121–130. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
