Meta-analysis of randomized controlled trials on efficacy and safety of extended thienopyridine therapy after drug-eluting stent implantation
- PMID: 27747163
- PMCID: PMC5059396
- DOI: 10.21037/cdt.2016.03.09
Meta-analysis of randomized controlled trials on efficacy and safety of extended thienopyridine therapy after drug-eluting stent implantation
Abstract
Background: The potential benefits and risks of extended thienopyridine therapy beyond 12 months after drug-eluting stent (DES) implantation remain unclear.
Methods: Randomized controlled trials (RCTs) were searched in PubMed, EMBASE, the Cochrane Library and China National Knowledge Infrastructure databases. The adverse clinical endpoints were compared between 12 months group (aspirin alone) and >12 months group (additional thienopyridine plus aspirin after 12-month dual antiplatelet therapy). Odds ratios (ORs) with 95% confidence intervals (95% CIs) were used as summary statistics. A random-effect model was used in the meta-analysis process.
Results: Finally, three RCTs incorporating 16,265 participants were included in this meta-analysis. The results indicated that the incidences of myocardial infarction (1.55% vs. 2.90%; OR =0.58; 95% CI, 0.40-0.84; P=0.004) and stent thrombosis (0.32% vs. 0.98%; OR =0.35; 95% CI, 0.20-0.62; P<0.001) in the >12 months group were significantly lower than the 12 months group. However, compared to the 12 months group, the extended thienopyridine therapy markedly increased the risk of bleeding events (2.09% vs. 1.28%; OR =1.64; 95% CI, 1.23-2.17; P<0.001). The risks of stroke (0.78% vs. 0.84%; P=0.67) and cardiac death (0.94% vs. 0.89%; P=0.61) were similar between the two groups.
Conclusions: The synthesis of available evidence indicates that a regimen of extended thienopyridine therapy beyond 12 months may significantly reduce the risks of myocardial infarction and stent thrombosis but increase the risk of bleeding events in the patients who have received DESs implantation.
Keywords: Drug-eluting stents (DESs); dual antiplatelet therapy; thienopyridine.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures







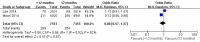



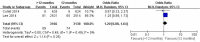



References
-
- Kolh P, Windecker S, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2014;46:517-92. 10.1093/ejcts/ezu366 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
