Detection and Management of Geographic Disparities in the TOPCAT Trial: Lessons Learned and Derivative Recommendations
- PMID: 27747305
- PMCID: PMC5065247
- DOI: 10.1016/j.jacbts.2016.03.001
Detection and Management of Geographic Disparities in the TOPCAT Trial: Lessons Learned and Derivative Recommendations
Abstract
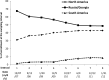
TOPCAT was a multinational clinical trial of 3,445 heart failure with preserved ejection fraction (HFpEF) patients that enrolled in 233 sites in six countries in North America, Eastern Europe and South America. Patients with a heart failure hospitalization in the last 12 months or an elevated B-type natriuretic peptide (BNP) were randomized to the mineralocorticoid receptor antagonist spironolactone vs. placebo. Sites in Russia and the Republic of Georgia provided the majority of early enrollment, primarily based on the hospitalization criterion since BNP levels were initially unavailable there. With the emergence of country-specific aggregate event rate data indicating lower rates in Eastern Europe and differences in patient characteristics there, the DSMB recommended relatively increasing enrollment in North America plus other corrective measures. Although final enrollment reflected the increased contribution from North America, a plurality of the final cohort came from Russia and Georgia (49% vs. 43% in North America). BNP measurements from Russia and Georgia available later in the trial suggested no or a mild level of heart failure consistent with low event rates. The primary results showed no significant spironolactone treatment effect overall (primary endpoint hazard ratio 0.89 (0.77, 1.04)), with a significant hazard ratio in North and South America (0.82 (0.69, 0.98), p =0.026) but not in Russia and Georgia (1.10 (0.79, 1.51), interaction p = 0.12). This report describes the DSMB's detection and management recommendations for regional differences in patient characteristics in TOPCAT, and suggests methods of surveillance and corrective actions that may be useful for future trials.
Figures


References
-
- Wedel H., Demets D., Deedwania P., for the MERIT-HF Study Group Challenges of subgroup analyses in multinational clinical trials: experiences from the MERIT-HF trial. Am Heart J. 2001;142:502–511. - PubMed
-
- O’Connor C.M., Fiuzat M., Swedberg K. Influence of global region on outcomes in large heart failure β-Blocker trials. J Am Coll Cardiol. 2011;58:915–922. - PubMed
-
- Pocock S., Calvo G., Marrugat J. International differences in treatment effect: do they really exist and why? Eur Heart J. 2013;34:1846–1852. - PubMed
-
- Glickman S.W., McHutchison J.G., Peterson E.D. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009;360:816–823. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

