Incidence of Sustained Ventricular Tachycardia in Patients with Prolonged QTc After the Administration of Azithromycin: A Retrospective Study
- PMID: 27747808
- PMCID: PMC4819483
- DOI: 10.1007/s40801-016-0062-9
Incidence of Sustained Ventricular Tachycardia in Patients with Prolonged QTc After the Administration of Azithromycin: A Retrospective Study
Abstract
Background: Azithromycin has been associated with abnormalities of cardiac repolarization and development of torsades de pointes. Observational data suggest that the risk of death from cardiovascular causes is increased in patients taking azithromycin. Little is known regarding the risk of ventricular arrhythmia in patients with prolongation of the corrected QT interval who receive azithromycin.
Objective: The purpose of this study was to determine the incidence of sustained ventricular tachycardia in patients with prolonged corrected QT (QTc) who subsequently received azithromycin.
Methods: We performed a retrospective cohort analysis of the incidence of sustained ventricular tachycardia in patients with prolonged QTc (greater than 450 ms) who successively received intravenous (IV) and/or oral azithromycin. Patients hospitalized in a tertiary care teaching hospital between November 2009 and June 2012 were included in the study. The primary outcome was sustained ventricular tachycardia documented in patients on telemetry.
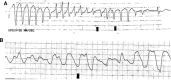
Results: Of the 103 patients enrolled in the study, only one patient experienced the primary outcome (0.97 %). The event occurred 1 day after the administration of a single dose of 500 mg IV azithromycin.
Conclusion: The risk of sustained ventricular tachycardia was 0.97 % in our cohort of patients with prolonged QTc who subsequently received azithromycin. Given the small size of this study, additional research is needed to determine the true incidence of arrhythmia in the population.
Conflict of interest statement
Author contributions
Steven Sears had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. Steven Sears, Trevor Getz, Christopher Austin, William Palmer, Evelyn Boyd, and Fernando Stancampiano contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Ethical approval
The study was approved by the Mayo Clinic Institutional Review Board, study ID 14-005155. It has been performed in accordance with the ethical standards of the declaration of Helsinki.
Funding
No sources of funding were used to assist in the preparation of this study.
Conflicts of interest
Steven P. Sears, Trevor W. Getz, Christopher O. Austin, William C. Palmer, Evelyn A. Boyd, and Fernando F. Stancampiano have no conflicts of interest and nothing to disclose.
Figures
References
-
- Inserts P. Zithromax (azithromycin for oral and injection) Package Inserts 4763, 5179.5191.
LinkOut - more resources
Full Text Sources
Other Literature Sources