Ergothioneine Biosynthesis and Functionality in the Opportunistic Fungal Pathogen, Aspergillus fumigatus
- PMID: 27748436
- PMCID: PMC5066259
- DOI: 10.1038/srep35306
Ergothioneine Biosynthesis and Functionality in the Opportunistic Fungal Pathogen, Aspergillus fumigatus
Abstract
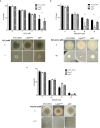
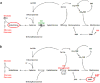
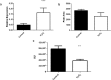
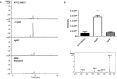
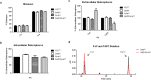
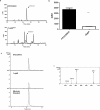
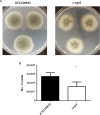
Ergothioneine (EGT; 2-mercaptohistidine trimethylbetaine) is a trimethylated and sulphurised histidine derivative which exhibits antioxidant properties. Here we report that deletion of Aspergillus fumigatus egtA (AFUA_2G15650), which encodes a trimodular enzyme, abrogated EGT biosynthesis in this opportunistic pathogen. EGT biosynthetic deficiency in A. fumigatus significantly reduced resistance to elevated H2O2 and menadione, respectively, impaired gliotoxin production and resulted in attenuated conidiation. Quantitative proteomic analysis revealed substantial proteomic remodelling in ΔegtA compared to wild-type under both basal and ROS conditions, whereby the abundance of 290 proteins was altered. Specifically, the reciprocal differential abundance of cystathionine γ-synthase and β-lyase, respectively, influenced cystathionine availability to effect EGT biosynthesis. A combined deficiency in EGT biosynthesis and the oxidative stress response regulator Yap1, which led to extreme oxidative stress susceptibility, decreased resistance to heavy metals and production of the extracellular siderophore triacetylfusarinine C and increased accumulation of the intracellular siderophore ferricrocin. EGT dissipated H2O2 in vitro, and elevated intracellular GSH levels accompanied abrogation of EGT biosynthesis. EGT deficiency only decreased resistance to high H2O2 levels which suggests functionality as an auxiliary antioxidant, required for growth at elevated oxidative stress conditions. Combined, these data reveal new interactions between cellular redox homeostasis, secondary metabolism and metal ion homeostasis.
Figures




Similar articles
-
A Single Aspergillus fumigatus Gene Enables Ergothioneine Biosynthesis and Secretion by Saccharomyces cerevisiae.Int J Mol Sci. 2022 Sep 16;23(18):10832. doi: 10.3390/ijms231810832. Int J Mol Sci. 2022. PMID: 36142753 Free PMC article.
-
SreA-mediated iron regulation in Aspergillus fumigatus.Mol Microbiol. 2008 Oct;70(1):27-43. doi: 10.1111/j.1365-2958.2008.06376.x. Epub 2008 Aug 21. Mol Microbiol. 2008. PMID: 18721228 Free PMC article.
-
SidL, an Aspergillus fumigatus transacetylase involved in biosynthesis of the siderophores ferricrocin and hydroxyferricrocin.Appl Environ Microbiol. 2011 Jul;77(14):4959-66. doi: 10.1128/AEM.00182-11. Epub 2011 May 27. Appl Environ Microbiol. 2011. PMID: 21622789 Free PMC article.
-
Involvement of Sulfur in the Biosynthesis of Essential Metabolites in Pathogenic Fungi of Animals, Particularly Aspergillus spp.: Molecular and Therapeutic Implications.Front Microbiol. 2019 Dec 13;10:2859. doi: 10.3389/fmicb.2019.02859. eCollection 2019. Front Microbiol. 2019. PMID: 31921039 Free PMC article. Review.
-
Resistance is not futile: gliotoxin biosynthesis, functionality and utility.Trends Microbiol. 2015 Jul;23(7):419-28. doi: 10.1016/j.tim.2015.02.005. Epub 2015 Mar 10. Trends Microbiol. 2015. PMID: 25766143 Review.
Cited by
-
Engineering the Yeast Saccharomyces cerevisiae for the Production of L-(+)-Ergothioneine.Front Bioeng Biotechnol. 2019 Oct 11;7:262. doi: 10.3389/fbioe.2019.00262. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31681742 Free PMC article.
-
H2S-Generating Cytosolic L-Cysteine Desulfhydrase and Mitochondrial D-Cysteine Desulfhydrase from Sweet Pepper (Capsicum annuum L.) Are Regulated During Fruit Ripening and by Nitric Oxide.Antioxid Redox Signal. 2023 Jul;39(1-3):2-18. doi: 10.1089/ars.2022.0222. Epub 2023 May 4. Antioxid Redox Signal. 2023. PMID: 36950799 Free PMC article.
-
Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues.Sci Rep. 2018 Jan 25;8(1):1601. doi: 10.1038/s41598-018-20021-z. Sci Rep. 2018. PMID: 29371632 Free PMC article.
-
50 Years of Organoselenium Chemistry, Biochemistry and Reactivity: Mechanistic Understanding, Successful and Controversial Stories.Chemistry. 2024 Dec 13;30(70):e202403003. doi: 10.1002/chem.202403003. Epub 2024 Nov 20. Chemistry. 2024. PMID: 39304519 Free PMC article. Review.
-
Ergothioneine, Ovothiol A, and Selenoneine-Histidine-Derived, Biologically Significant, Trace Global Alkaloids.Molecules. 2022 Apr 21;27(9):2673. doi: 10.3390/molecules27092673. Molecules. 2022. PMID: 35566030 Free PMC article. Review.
References
-
- Carlsson J., Kierstan M. P. & Brocklehurst K. Reactions of L-ergothioneine and some other aminothiones with2,2′-and 4,4′-dipyridyl disulphides and of L-ergothioneine with iodoacetamide. 2-Mercaptoimidazoles, 2- and 4-thiopyridones, thiourea and thioacetamide as highly reactive neutral sulphur nucleophils. Biochem. J. 139, 221–235 (1974). - PMC - PubMed
-
- Jocelyn P. In Biochemistry of the SH Group. The Occurrence, Chemical Properties, Metabolism and Biological Functions of Thiols and Disulphides. (Academic Press, 1972).
-
- Fahey R. C. Glutathione analogs in prokaryotes. Biochimica et Biophysica Acta (BBA)-General Subjects 1830, 3182–3198 (2013). - PubMed
-
- Jones G. W., Doyle S. & Fitzpatrick D. A. The evolutionary history of the genes involved in the biosynthesis of the antioxidant ergothioneine. Gene 549, 161–170 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

