Effect of endotoxemia in mice genetically deficient in cystathionine-γ-lyase, cystathionine-β-synthase or 3-mercaptopyruvate sulfurtransferase
- PMID: 27748832
- PMCID: PMC5117757
- DOI: 10.3892/ijmm.2016.2771
Effect of endotoxemia in mice genetically deficient in cystathionine-γ-lyase, cystathionine-β-synthase or 3-mercaptopyruvate sulfurtransferase
Abstract
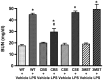
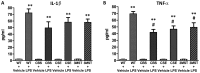
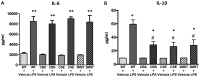
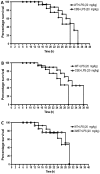
Hydrogen sulfide (H2S) has been proposed to exert pro- as well as anti-inflammatory effects in various models of critical illness. In this study, we compared bacterial lipopolysaccharide (LPS)‑induced changes in inflammatory mediator production, indices of multiple organ injury and survival in wild‑type (WT) mice and in mice with reduced expression of one of the three H2S‑producing enzymes, cystathionine-γ-lyase (CSE), cystathionine-β-synthase (CBS) or 3-mercaptopyruvate sulfurtransferase (3MST). Mice were injected intraperitoneally (i.p.) with LPS (10 mg/kg). After 6 h, the animals were sacrificed, blood and organs were collected and the following parameters were evaluated: blood urea nitrogen (BUN) levels in blood, myeloperoxidase (MPO) and malondialdehyde (MDA) in the lung, cytokine levels in plasma and the expression of the three H2S‑producing enzymes (CBS, CSE and 3MST) in the spleen, lung, liver and kidney. LPS induced a tissue‑dependent upregulation of some of the H2S‑producing enzymes in WT mice (upregulation of CBS in the spleen, upregulation of 3MST in the liver and upregulation of CBS, CSE and 3MST in the lung). Moreover, LPS impaired glomerular function, as evidenced by increased BUN levels. Renal impairment was comparable in the CSE‑/‑ and Δ3MST mice after LPS challenge; however, it was attenuated in the CBS+/‑ mice. MPO levels (an index of neutrophil infiltration) and MDA levels (an index of oxidative stress) in lung homogenates were significantly increased in response to LPS; these effects were similar in the WT, CBS+/‑, CSE‑/‑ and Δ3MST mice; however, the MDA levels tended to be lower in the CBS+/‑ and CSE‑/‑ mice. LPS induced significant increases in the plasma levels of multiple cytokines [tumor necrosis factor (TNF)α, interleukin (IL)‑1β, IL‑6, IL‑10, IL‑12 and interferon (IFN)γ] in plasma; TNFα, IL‑10 and IL‑12 levels tended to be lower in all three groups of animals expressing lower levels of H2S‑producing enzymes. The survival rates after the LPS challenge did not show any significant differences between the four animal groups tested. Thus, the findings of this study indicate that a deficiency in 3MST does not significantly affect endotoxemia, while a deficiency in CBS or CSE slightly ameliorates the outcome of LPS-induced endotoxemia in vivo.
Figures


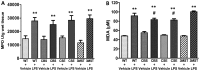


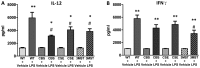
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

