Nasal decongestants in monotherapy for the common cold
- PMID: 27748955
- PMCID: PMC6461189
- DOI: 10.1002/14651858.CD009612.pub2
Nasal decongestants in monotherapy for the common cold
Abstract
Background: Many treatments for the common cold exist and are sold over-the-counter. Nevertheless, evidence on the effectiveness and safety of nasal decongestants is limited.
Objectives: To assess the efficacy, and short- and long-term safety, of nasal decongestants used in monotherapy to alleviate symptoms of the common cold in adults and children.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 6, June 2016), which contains the Cochrane Acute Respiratory Infections (ARI) Specialised Register, MEDLINE (1946 to July 2016), Embase (2010 to 15 July 2016), CINAHL (1981 to 15 July 2016), LILACS (1982 to July 2016), Web of Science (1955 to July 2016) and clinical trials registers.
Selection criteria: Randomised controlled trials (RCTs) and cluster-RCTs investigating the effectiveness and adverse effects of nasal decongestants compared with placebo for treating the common cold in adults and children. We excluded quasi-RCTs.
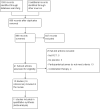
Data collection and analysis: Three review authors independently extracted and summarised data on subjective measures of nasal congestion, overall patient well-being score, objective measures of nasal airway resistance, adverse effects and general recovery. One review author acted as arbiter in cases of disagreement. We categorised trials as single and multi-dose and analysed data both separately and together. We also analysed studies using an oral or topical nasal decongestant separately and together.
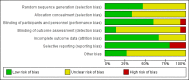
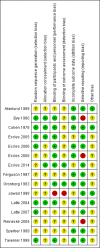
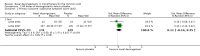
Main results: We included 15 trials with 1838 participants. Fourteen studies included adult participants only (aged 18 years and over). In six studies the intervention was a single dose and in nine studies multiple doses were used. Nine studies used pseudoephedrine and three studies used oxymetazoline. Other decongestants included phenylpropanolamine, norephedrine and xylometazoline. Phenylpropanolamine (or norephedrine) is no longer available on the market therefore we did not include the results of these studies in the meta-analyses. Eleven studies used oral decongestants; four studies used topical decongestants.Participants were included after contracting the common cold. The duration of symptoms differed among studies; in 10 studies participants had symptoms for less than three days, in three studies symptoms were present for less than five days, one study counted the number of colds over one year, and one study experimentally induced the common cold. In the single-dose studies, the effectiveness of a nasal decongestant was measured on the same day, whereas the follow-up in multi-dose studies ranged between one and 10 days.Most studies were conducted in university settings (N = eight), six at a specific university common cold centre. Three studies were conducted at a university in collaboration with a hospital and two in a hospital only setting. In two studies the setting was unclear.There were large differences in the reporting of outcomes and the reporting of methods in most studies was limited. Therefore, we judged most studies to be at low or unclear risk of bias. Pooling was possible for a limited number of studies only; measures of effect are expressed as standardised mean differences (SMDs). A positive SMD represents an improvement in congestion. There is no defined minimal clinically important difference for measures of subjective improvement in nasal congestion, therefore we used the SMDs as a guide to assess whether an effect was small (0.2 to 0.49), moderate (0.5 to 0.79) or large (≥ 0.8).Single-dose decongestant versus placebo: 10 studies compared a single dose of nasal decongestant with placebo and their effectiveness was tested between 15 minutes and 10 hours after dosing. Seven of 10 studies reported subjective symptom scores for nasal congestion; none reported overall patient well-being. However, pooling was not possible due to the large diversity in the measurement and reporting of symptoms of congestion. Two studies recorded adverse events. Both studies used an oral decongestant and each of them showed that there was no statistical difference between the number of adverse events in the treatment group versus the placebo group.Multi-dose decongestant versus placebo: nine studies compared multiple doses of nasal decongestants with placebo, but only five reported on the primary outcome, subjective symptom scores for nasal congestion. Only one study used a topical decongestant; none reported overall patient well-being. Subjective measures of congestion were significantly better for the treatment group compared with placebo approximately three hours after the last dose (SMD 0.49, 95% confidence interval (CI) 0.07 to 0.92; P = 0.02; GRADE: low-quality evidence). However, the SMD of 0.49 only indicates a small clinical effect. Pooling was based on two studies, one oral and one topical, therefore we were unable to assess the effects of oral and topical decongestants separately. Seven studies reported adverse events (six oral and one topical decongestant); meta-analysis showed that there was no statistical difference between the number of adverse events in the treatment group (125 per 1000) compared to the placebo group (126 per 1000). The odds ratio (OR) for adverse events in the treatment group was 0.98 (95% CI 0.68 to 1.40; P = 0.90; GRADE: low-quality evidence). The results remained the same when we only considered studies using an oral decongestant (OR 0.95, 95% CI 0.65 to 1.39; P = 0.80; GRADE: low-quality evidence).
Authors' conclusions: We were unable to draw conclusions on the effectiveness of single-dose nasal decongestants due to the limited evidence available. For multiple doses of nasal decongestants, the current evidence suggests that these may have a small positive effect on subjective measures of nasal congestion in adults with the common cold. However, the clinical relevance of this small effect is unknown and there is insufficient good-quality evidence to draw any firm conclusions. Due to the small number of studies that used a topical nasal decongestant, we were also unable to draw conclusions on the effectiveness of oral versus topical decongestants. Nasal decongestants do not seem to increase the risk of adverse events in adults in the short term. The effectiveness and safety of nasal decongestants in children and the clinical relevance of their small effect in adults is yet to be determined.
Conflict of interest statement
Laura Deckx: none known An IM De Sutter: none known Linda Guo: none known Nabiel A Mir: none known Mieke L van Driel: none known
Figures







Update of
- doi: 10.1002/14651858.CD009612
References
References to studies included in this review
Akerlund 1989 {published data only}
-
- Akerlund A, Klint T, Olen L, Rundcrantz H. Nasal decongestant effect of oxymetazoline in the common cold: an objective dose‐response study in 106 patients. Journal of Laryngology & Otology 1989;103(8):743‐6. - PubMed
Bye 1980 {published data only}
Cohen 1978 {published data only}
-
- Cohen BM. Clinical correlants of changes in nasal flow/resistance (Rn) measurements. Allergologia et Immunopathologia 1978;6(3):217‐23. - PubMed
Eccles 2005 {published data only}
-
- Eccles R, Jawad MSM, Jawad SSM, Angello JT, Druce HM. Efficacy and safety of single and multiple doses of pseudoephedrine in the treatment of nasal congestion associated with common cold. American Journal of Rhinology 2005;19(1):25‐31. - PubMed
Eccles 2006 {published data only}
-
- Eccles R, Jawad M, Jawad S, Ridge D, North M, Jones E, et al. Efficacy of a paracetamol‐pseudoephedrine combination for treatment of nasal congestion and pain‐related symptoms in upper respiratory tract infection. Current Medical Research and Opinion 2006;22(12):2411‐8. - PubMed
Eccles 2008 {published data only}
-
- Eccles R, Eriksson M, Garreffa S, Chen SC. The nasal decongestant effect of xylometazoline in the common cold. American Journal of Rhinology 2008;22(5):491‐6. - PubMed
-
- Eccles R, Martensson K, Stephen G, Chen S. A double‐blind, randomised, parallel group, placebo‐controlled study evaluating the nasal decongestant effect of xylometazoline in common cold [Abstract]. Primary Care Respiratory Journal 2008;17(2):120.
Eccles 2014 {published data only}
Ferguson 1997 {published data only}
-
- Ferguson EA, Eccles R. Changes in nasal nitric oxide concentration associated with symptoms of common cold and treatment with a topical nasal decongestant. Acta Oto‐Laryngologica 1997;117(4):614‐7. - PubMed
Gronborg 1983 {published data only}
-
- Gronborg H, Winther B, Brofeldt S, Borum P, Mygind N. Effects of oral norephedrine on common cold symptoms. Rhinology 1983;21(1):3‐12. - PubMed
Jawad 1998 {published data only}
-
- Jawad SS, Eccles R. Effect of pseudoephedrine on nasal airflow in patients with nasal congestion associated with common cold. Rhinology 1998;36(2):73‐6. - PubMed
Latte 2004 {published data only}
-
- Latte J, Taverner D, Slobodian P, Shakib S. A randomized, double‐blind, placebo‐controlled trial of pseudoephedrine in coryza. Clinical and Experimental Pharmacology & Physiology 2004;31(7):429‐32. - PubMed
Latte 2007 {published data only}
-
- Latte J, Taverner D. Clinical trial of 3 days of treatment with oral pseudoephedrine for the common cold in the southern hemisphere. American Journal of Rhinology 2007;21(4):452‐5. - PubMed
Reinecke 2005 {published data only}
-
- Reinecke S, Tschaikin M. Investigation of the effect of oxymetazoline on the duration of rhinitis [Alpha‐Sympathomimetikum bessert nicht nur die nasale Obstruktion: Schnupfendauer wird verkürzt]. MMW Fortschritte der Medizin 2005;147(41):46. - PubMed
Sperber 1989 {published data only}
Taverner 1999 {published data only}
-
- Taverner D, Danz C, Economos D. The effects of oral pseudoephedrine on nasal patency in the common cold: a double‐blind single‐dose placebo‐controlled trial. Clinical Otolaryngology and Allied Sciences 1999;24(1):47‐51. - PubMed
References to studies excluded from this review
Ackerhans 1994 {published data only}
-
- Ackerhans M, Jannert M, Tonnesson M. Effects of a new administration form of oxymetazoline on maxillary ostial patency in healthy individuals and patients with acute rhinitis. Acta Oto‐Laryngologica Supplement 1994;515:49‐52. - PubMed
Anderson 1956 {published data only}
-
- Anderson HA. Clinical trial of tetrahydrozoline pediatric nasal decongestant. Antibiotic Medicine & Clinical Therapy 1956;3(3):199‐201. - PubMed
Anonymous 1975 {published data only}
-
- Anonymous. Evaluation of a new oxymetazoline preparation in the treatment of nasal congestion. A multicentre trial. Practitioner 1975;214(1283):685‐8. - PubMed
Ashe 1968 {published data only}
-
- Ashe GJ. Oral medications in nasal decongestion. A study among industrial workers. Industrial Medicine and Surgery 1968;37(3):212‐4. - PubMed
Bailey 1969 {published data only}
-
- Bailey BJ. Clinical evaluation of a topical nasal decongestant ‐ oxymetazoline. Eye, Ear, Nose & Throat Monthly 1969;48(9):512‐5. - PubMed
Bende 1984 {published data only}
-
- Bende M, Andersson KE, Johansson CJ, Sjögren C, Svensson G. Dose‐response relationship of a topical nasal decongestant: phenylpropanolamine. Acta Oto‐Laryngologica 1984;98(5‐6):543‐7. - PubMed
Bende 1985 {published data only}
-
- Bende M. Contralateral effects of unilateral nasal decongestion. Acta Oto‐Laryngologica 1985;99(5‐6):637‐40. - PubMed
Broms 1982 {published data only}
-
- Broms P, Malm L. Oral vasoconstrictors in perennial non‐allergic rhinitis. Allergy 1982;37(2):67‐74. - PubMed
Castellano 2002 {published data only}
-
- Castellano F, Mautone G. Decongestant activity of a new formulation of xylometazoline nasal spray: a double‐blind, randomized versus placebo and reference drugs controlled, dose‐effect study. Drugs Under Experimental & Clinical Research 2002;28(1):27‐35. - PubMed
Cohen 1977 {published data only}
-
- Cohen BM. Physiologic and subjective comparisons of 2 oral sustained‐release nasal decongestant combinations and placebo in patients with common colds. Current Therapeutic Research 1977;22(4):522‐8.
Connell 1969 {published data only}
-
- Connell JT. Effectiveness of topical nasal decongestants. Annals of Allergy 1969;27(11):541‐6. - PubMed
De Paula Neves 1966 {published data only}
-
- Paula Neves F. Clinical trial of a drug combination (JJ‐4482) used for the treatment of catarrhal inflammations of the upper respiratory tract [Ensaio clinico com uma associacao medicamentosa (JJ‐4482) destinada ao tratamento das inflamacoes catarrais das vias aereas superiores]. Revista Brasileira de Medicina 1966;23(12):878‐80. - PubMed
Dorn 2003 {published data only}
-
- Dorn M, Hofmann W, Knick E. Tolerance and effectiveness of oxymetazoline and xylometazoline in treatment of acute rhinitis [Verträglichkeit und Wirksamkeit von Oxymetazolin* und Xylometazolin bei der Behandlung der akuten Rhinitis]. HNO 2003;51(10):794‐9. - PubMed
Fox 1967 {published data only}
-
- Fox SL. The use of oral decongestants in the management of diverse types of nasal congestion. Current Therapeutic Research 1967;9(5):247‐52. - PubMed
Hummel 1998 {published data only}
-
- Hummel T, Rothbauer C, Pauli E, Kobal G. Effects of the nasal decongestant oxymetazoline on human olfactory and intranasal trigeminal function in acute rhinitis. European Journal of Clinical Pharmacology 1998;54(7):521‐8. - PubMed
Katrana 1956 {published data only}
-
- Katrana NJ. Clinical trial of tyzine, a new nasal decongestant. Illinois Medical Journal 1956;110(1):19‐21. - PubMed
McElhenney 1966 {published data only}
-
- McElhenney TR, Grater WC, Pfeiffer GO, Sanders SH, McGovern JP, Weisberg J. A comparative evaluation of four oral nasal decongestants. Current Therapeutic Research 1966;8(6):291‐8. - PubMed
Meurman 1975 {published data only}
-
- Meurman OH. A controlled clinical comparison of nasal decongestants in acute rhinitis. Journal of International Medical Research 1975;3(5):356‐62.
Pritchard 2014 {published data only}
-
- Pritchard S, Glover M, Guthrie G, Brum J, Ramsey D, Kappler G, et al. Effectiveness of 0.05% oxymetazoline (Vicks Sinex Micromist®) nasal spray in the treatment of objective nasal congestion demonstrated to 12 h post‐administration by magnetic resonance imaging. Pulmonary Pharmacology and Therapeutics 2014;27(1):121‐6. - PubMed
Rumiantsev 1993 {published data only}
-
- Rumiantsev AG, Aldonina VV, Il'ina TP, Snitarenko VI, Biriuchinskaia IG, Shevaldina TV. A trial of the use of the prolonged‐action preparation Koldakt from the Natko firm for treating upper respiratory tract diseases in polyclinics [Opyt primeneniia preparata prolongirovannogo deistviia "Koldakt" firmy "Natko" dlia lecheniia zabolevanii verkhnikh dykhatel'nykh putei v poliklinicheskikh usloviiakh]. Terapevticheskii Arkhiv 1993;65(11):70, 72. - PubMed
Smith 1999 {published data only}
-
- Smith A, Sturgess W, Rich N, Brice C, Collison C, Bailey J, et al. The effects of idazoxan on reaction times, eye movements and the mood of healthy volunteers and patients with upper respiratory tract illnesses. Journal of Psychopharmacology 1999;13(2):148‐51. - PubMed
Tzachev 2002 {published data only}
Weisberg 1966 {published data only}
-
- Weisberg J, Breslow I. The evaluation of a nasal decongestant preparation. Archives of Otolaryngology 1966;83(5):477‐9. - PubMed
Winther 1983 {published data only}
-
- Winther B, Brofeldt S, Borum P, Pedersen M, Mygind N. Lack of effect on nasal discharge from a vasoconstrictor spray in common cold. European Journal of Respiratory Diseases Supplement 1983;128(Pt 2):447‐8. - PubMed
Zumpft 1975 {published data only}
-
- Zumpft CW. Double‐blind comparison of metizoline hydrochloride (Ellsyl) and phenylephrine in allergic and vasomotor rhinitis. Current Therapeutic Research 1975;17(1):40‐6. - PubMed
References to studies awaiting assessment
NCT00452270 {unpublished data only}
-
- NCT00452270. A double‐blind, randomized, parallel group, placebo controlled study, evaluating the decongestant effect, time to onset, duration of effect and impact on sleep and general well‐being of xylometazoline in subjects with a common cold. https://clinicaltrials.gov/ct2/show/NCT00452270 (accessed 6 October 2015).
NCT01062360 {unpublished data only}
-
- NCT01062360. A pivotal, placebo controlled, phase III study to compare efficacy and tolerability of a fixed combination, containing 500 mg ASA and 30 mg pseudoephedrine, in comparison to its single components in patients with sore throat and nasal congestion. https://clinicaltrials.gov/ct2/show/NCT01062360 (accessed 6 October 2015).
References to ongoing studies
EUCTR2006‐006690‐25‐GB {unpublished data only}
-
- EUCTR2006‐006690‐25‐GB. A double‐blind, randomized, parallel group, placebo controlled study, evaluating the decongestant effect, time to onset, duration of effect and impact on sleep and general well‐being of otrivin F2 in subjects with a common cold ‐ a study to evaluate the decongestant effect of Otrivin F2. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2006‐006690‐25/GB (accessed 6 October 2015).
NCT01744106 {unpublished data only}
-
- NCT01744106. A multicenter study of pseudoephedrine for the temporary relief of nasal congestion in children with the common cold. https://clinicaltrials.gov/ct2/show/NCT01744106 (accessed 6 October 2015). - PMC - PubMed
Additional references
AlBalawi 2013
Allan 2014
Arroll 2005
-
- Arroll B. Non‐antibiotic treatments for upper‐respiratory tract infections (common cold). Respiratory Medicine 2005;99:1477‐84. - PubMed
Arroll 2011
-
- Arroll B. Common cold. BMJ Clinical Evidence 2011. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3275147/pdf/2011‐1510.pdf (accessed 3 November 2015).
Atkins 2004
De Sutter 2012
Del Mar 2003
-
- Mar C, Glasziou P. Upper respiratory tract infection. BMJ Clinical Evidence 2003;10:1747‐56. - PubMed
Dolansky 2008
Eccles 2000
-
- Eccles R. Pathophysiology of nasal symptoms. American Journal of Rhinology 2000;14(5):335‐8. - PubMed
Eccles 2007
Eccles 2009
-
- Eccles R. Over the counter medicines for colds. In: Eccles R, Webber O editor(s). Common Cold. Basel: Birkhauser Verlag, 2009:23‐45.
Eccles 2010
-
- Eccles R, Martensson K, Chen SC. Effects of intranasal xylometazoline, alone or in combination with ipratropium, in patients with common cold. Current Medical Research & Opinion 2010;26:889‐99. - PubMed
FDA 2000
-
- FDA Public Health Advisory: Safety of Phenylpropanolamine. Federal Register 2012. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPa... (accessed 22 April 2016).
FDA 2005
-
- Information by drug class: Legal Requirements for the Sale and Purchase of Drug Products Containing Pseudoephedrine, Ephedrine, and Phenylpropanolamine. Federal Register 2005. http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm072423.htm (accessed 22 April 2016).
FDA 2012
-
- FDA Drug Safety Communication: Serious adverse events from accidental ingestion by children of over‐the‐counter eye drops and nasal sprays. Federal Register 2012. http://www.fda.gov/Drugs/DrugSafety/ucm325257.htm (accessed 3 November 2015).
Fendrick 2003
-
- Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non‐influenza‐related viral respiratory tract infection in the United States. Archives of Internal Medicine 2003;163(4):487–94. - PubMed
Fry 1993
-
- Fry J, Sandler G. Common Diseases. Their Nature, Prevalence and Care. 5th Edition. Dordrecht: Kluwer Academic, 1993.
GRADEproGDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc.). GRADEproGDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Hayward 2015
Heikkinnenn 2003
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
King 2015
Kollar 2007
-
- Kollar C, Schneider H, Waksman J, Krusinska E. Meta‐analysis of the efficacy of a single dose of phenylephrine 10 mg compared with placebo in adults with acute nasal congestion due to the common cold. Clinical Therapeutics 2007;29:1057‐70. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J (editors). Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Meltzer 2010
Moher 2009
NPS Medicinewise 2012
-
- NPS Medicinewise. Nasal decongestants. National Prescribing Service (http://www.nps.org.au/medicines/ear‐nose‐mouth‐and‐throat/nasal‐deconges...) (accessed 9 July 2016) 2012. [http://www.nps.org.au/medicines/ear‐nose‐mouth‐and‐throat/nasal‐deconges...
Pappas 2015
-
- Pappas D. The common cold in children. Available from www.uptodate.com 2015.
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2009
-
- Schünemann H, Brozek J, Oxman A (editors). GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2 [updated March 2009]. The GRADE Working Group. Available from http://www.cc‐ims.net/gradepro 2009.
Simasek 2007
-
- Simasek M, Blandino D. Treatment of the common cold. American Family Physician 2007;75(4):515‐20. - PubMed
Singh 2013
Sterne 2011
-
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Wicker 2009
-
- Wicker A, Labruzzo B. Recommendations for the use of OTC cough and cold medications in children: decongestants. US Pharmacist 2009;34(3):33‐6.
References to other published versions of this review
Ta'i 2012
-
- Ta'i SH, Ferguson KAM, Singh HK, Sharma AN, Kumar S, Driel ML, et al. Nasal decongestants for the common cold. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009612] - DOI
Taverner 2004
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

