Efficacy and Safety of Bevacizumab-Containing Therapy in Newly Diagnosed Ovarian Cancer: ROSiA Single-Arm Phase 3B Study
- PMID: 27749456
- PMCID: PMC5181120
- DOI: 10.1097/IGC.0000000000000836
Efficacy and Safety of Bevacizumab-Containing Therapy in Newly Diagnosed Ovarian Cancer: ROSiA Single-Arm Phase 3B Study
Abstract
Objective: The aim of this study was to assess the safety and efficacy of extending bevacizumab therapy beyond 15 months in nonprogressive ovarian cancer.
Patients and methods: In this multinational prospective single-arm study (ClinicalTrials.gov NCT01239732), eligible patients had International Federation of Gynecology and Obstetrics stage IIB to IV or grade 3 stage I to IIA ovarian cancer without clinical signs or symptoms of gastrointestinal obstruction or history of abdominal fistula, gastrointestinal perforation, or intra-abdominal abscess within the preceding 6 months. Prior neoadjuvant chemotherapy was permitted. After debulking surgery, patients received bevacizumab 15 (or 7.5) mg/kg every 3 weeks (q3w) with 4 to 8 cycles of paclitaxel (investigator's choice of 175 mg/m q3w or 80 mg/m weekly) plus carboplatin AUC 5 to 6 q3w. Single-agent bevacizumab was continued until progression or for up to 24 months. The primary end point was safety.
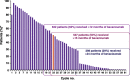
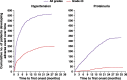
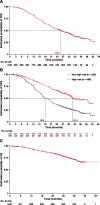
Results: Between December 2010 and May 2012, 1021 patients from 35 countries began study treatment. Bevacizumab was administered at 15 mg/kg in 89% of patients and for more than 15 months in 53%. Median follow-up duration was 32 months (range, 1-50 months). The most common all-grade adverse events were hypertension (55% of patients), neutropenia (49%), and alopecia (43%). The most common grade 3 or higher-grade adverse events were neutropenia (27%) and hypertension (25%). Bevacizumab was discontinued because of proteinuria in 5% of patients and hypertension in 3%. Median progression-free survival (PFS) was 25.5 months (95% confidence interval, 23.7-27.6 months).
Conclusion: Extended bevacizumab demonstrated increased incidences of proteinuria and hypertension compared with 12 or 15 months of bevacizumab in previous trials, but these rarely led to bevacizumab discontinuation. Median PFS is the longest reported for frontline bevacizumab-containing therapy. The longer bevacizumab duration beyond 15 months in this study may improve PFS without substantially compromising safety.
Figures
References
-
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. - PubMed
-
- Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. - PubMed
-
- Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. - PubMed
-
- Randall L, Burger R, Nguyen H, et al. Outcome differences in patients with advanced epithelial ovarian, primary peritoneal and fallopian tube cancers treated with and without bevacizumab. Gynecol Oncol. 2013;130:e33 (Abstract 80).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

