Safety and Efficacy of Atorvastatin in Human Immunodeficiency Virus-infected Children, Adolescents and Young Adults With Hyperlipidemia
- PMID: 27749649
- PMCID: PMC5154931
- DOI: 10.1097/INF.0000000000001352
Safety and Efficacy of Atorvastatin in Human Immunodeficiency Virus-infected Children, Adolescents and Young Adults With Hyperlipidemia
Abstract
Background: Human immunodeficiency virus (HIV)-infected children receiving antiretroviral therapy (ART) have increased prevalence of hyperlipidemia and risk factors for cardiovascular disease. No studies have investigated the efficacy and safety of statins in this population.
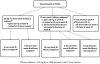
Methods: HIV-infected youth 10 to <24 years of age on stable ART with low-density lipoprotein cholesterol (LDL-C) ≥130 mg/dL for ≥6 months initiated atorvastatin 10 mg once daily. Atorvastatin was increased to 20 mg if LDL-C efficacy criteria (LDL-C < 110 mg/dL or decreased ≥30% from baseline) were not met at week 4. Primary outcomes were safety and efficacy.
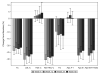
Results: Twenty-eight youth initiated atorvastatin; 7 were 10-15 years and 21 were 15-24 years. Mean baseline LDL-C was 161 mg/dL (standard deviation 19 mg/dL). Efficacy criteria were met at week 4 by 17 of 27 (63%) participants. Atorvastatin was increased to 20 mg in 10 participants. Mean LDL-C decreased from baseline by 30% (90% confidence interval: 26%, 35%) at week 4, 28% (90% confidence interval: 23%, 33%) at week 24 and 26% (90% confidence interval: 20%, 33%) at week 48. LDL-C was less than 110 mg/dL in 44% at week 4, 42% at week 12 and 46% at weeks 24 and 48. Total cholesterol, non high-density lipoprotein (non-HDL)-C and apolipoprotein B decreased significantly, but IL-6 and high-sensitivity C-reactive protein did not. Two participants in the younger age group discontinued study for toxicities possibly related to atorvastatin.
Conclusions: Atorvastatin lowered total cholesterol, LDL-C, non HDL-C and apolipoprotein B in HIV-infected youth with ART-associated hyperlipidemia. Atorvastatin could be considered for HIV-infected children with hyperlipidemia, but safety monitoring is important particularly in younger children.
Conflict of interest statement
No authors report conflicts of interest.
Figures
References
-
- Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

