Intratympanic Sustained-Exposure Dexamethasone Thermosensitive Gel for Symptoms of Ménière's Disease: Randomized Phase 2b Safety and Efficacy Trial
- PMID: 27749754
- PMCID: PMC5414596
- DOI: 10.1097/MAO.0000000000001227
Intratympanic Sustained-Exposure Dexamethasone Thermosensitive Gel for Symptoms of Ménière's Disease: Randomized Phase 2b Safety and Efficacy Trial
Abstract
Objective: To evaluate safety and efficacy of a single intratympanic injection of OTO-104, sustained-exposure dexamethasone, in patients with unilateral Ménière's disease.
Study design: Randomized, double-blind, placebo-controlled, Phase 2b study over 5 months.
Setting: Fifty-two academic and community otolaryngology centers.
Patients: One hundred fifty four patients (77 per group) aged 18 to 85 years inclusive.
Intervention: Single intratympanic injection of OTO-104 (12 mg dexamethasone) or placebo.
Main outcome measures: Efficacy (vertigo) and safety (adverse events, otoscopy, audiometry, tympanometry).
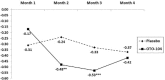
Results: Primary endpoint (change from baseline in vertigo rate at Month 3) was not statistically significant (placebo [-43%], OTO-104 [-61%], P = 0.067). Improvements with OTO-104 were observed in prospectively defined secondary endpoints number of days with definitive vertigo, (Month 2 [P = 0.035], Month 3 [P = 0.030]), vertigo severity (Months 2-3, P = 0.046) and daily vertigo counts (Month 2, P = 0.042), and in some Short Form-36 (SF-36) subscales (Month 2 bodily pain P = 0.039, vitality P = 0.045, social functioning P = 0.025). No difference in tinnitus loudness or tinnitus handicap inventory (THI-25) was observed. OTO-104 was well tolerated; no negative impact on safety compared with placebo. Persistent tympanic membrane perforation was observed in two OTO-104 treated patients at study end.
Conclusion: OTO-104 was well-tolerated, did not significantly affect change from baseline in vertigo rate, but did reduce number definitive vertigo days, vertigo severity, and average daily vertigo count compared with placebo during Month 3. Results provide insight into analyzing for a vertigo treatment effect and support advancing OTO-104 into Phase 3 clinical trials for the treatment of Ménière's disease symptoms.
Figures




References
-
- Barritt L. Ménière's disease. XPharm: Compr Pharmacol Ref 2008; 3:1–6.
-
- Committee on Hearing and Equilibrium. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Ménière's disease. Otol-Head Neck Surg 1995; 113:181–185. - PubMed
-
- Shea JJ. Classification of Ménière's disease. Otol Neuro 1993; 14:224–229. - PubMed
-
- Paparella MM. Pathogenesis of Ménière's disease and Ménière's syndrome. Acta Otolaryngol Suppl 1984; 406:10–25. - PubMed
-
- Anderson JP, Harris JP. Impact of Ménière's disease on quality of life. Otol Neurotol 2001; 22:888–894. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

