Safety and Immunogenicity of Pfs25-EPA/Alhydrogel®, a Transmission Blocking Vaccine against Plasmodium falciparum: An Open Label Study in Malaria Naïve Adults
- PMID: 27749907
- PMCID: PMC5066979
- DOI: 10.1371/journal.pone.0163144
Safety and Immunogenicity of Pfs25-EPA/Alhydrogel®, a Transmission Blocking Vaccine against Plasmodium falciparum: An Open Label Study in Malaria Naïve Adults
Abstract
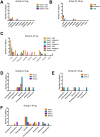
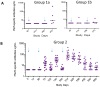
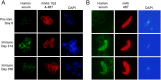
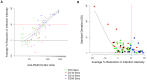
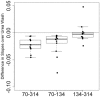
Transmission-blocking vaccines (TBVs) that target sexual stage parasite development could be an integral part of measures for malaria elimination. Pfs25 is a leading TBV candidate, and previous studies conducted in animals demonstrated an improvement of its functional immunogenicity after conjugation to EPA, a recombinant, detoxified ExoProtein A from Pseudomonas aeruginosa. In this report, we describe results of an open-label, dose-escalating Phase 1 trial to assess the safety and immunogenicity of Pfs25-EPA conjugates formulated with Alhydrogel®. Thirty malaria-naïve healthy adults received up to four doses of the conjugate vaccine, with 8, 16, or 47 μg of conjugated Pfs25 mass, at 0, 2, 4, and 10 months. Vaccinations were generally well tolerated. The majority of solicited adverse events were mild in severity with pain at the injection site the most common complaint. Anemia was the most common laboratory abnormality, but was considered possibly related to the study in only a minority of cases. No vaccine-related serious adverse events occurred. The peak geometric mean anti-Pfs25 antibody level in the highest dose group was 88 (95% CI 53, 147) μg/mL two weeks after the 4th vaccination, and declined to near baseline one year later. Antibody avidity increased over successive vaccinations. Transmission blocking activity demonstrated in a standard membrane feeding assay (SMFA) also increased from the second to the third dose, and correlated with antibody titer and, after the final dose, with antibody avidity. These results support the further evaluation of Pfs25-EPA/Alhydrogel® in a malaria-endemic population.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- WHO. World Malaria Report 2015. Geneva: WHO, 2016.
-
- Group MVF. Malaria Vaccine Technology Roadmap. 2013.
-
- Group MVF. Malaria Vaccine Technology Roadmap. 2006.
-
- First malaria vaccine receives positive scientific opinion from EMA [Internet]. 2015. Mosquirix to be used for vaccination of young children, together with established antimalarial interventions; 24 July 2015. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

