Insulin secretagogues for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus
- PMID: 27749986
- PMCID: PMC6461156
- DOI: 10.1002/14651858.CD012151.pub2
Insulin secretagogues for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus
Abstract
Background: The projected rise in the incidence of type 2 diabetes mellitus (T2DM) could develop into a substantial health problem worldwide. Whether insulin secretagogues (sulphonylureas and meglitinide analogues) are able to prevent or delay T2DM and its associated complications in people at risk for the development of T2DM is unknown.
Objectives: To assess the effects of insulin secretagogues on the prevention or delay of T2DM and its associated complications in people with impaired glucose tolerance, impaired fasting blood glucose, moderately elevated glycosylated haemoglobin A1c (HbA1c) or any combination of these.
Search methods: We searched the Cochrane Central Register of Controlled Trials, MEDLINE, PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform, and the reference lists of systematic reviews, articles and health technology assessment reports. We asked investigators of the included trials for information about additional trials. The date of the last search of all databases was April 2016.
Selection criteria: We included randomised controlled trials (RCTs) with a duration of 12 weeks or more comparing insulin secretagogues with any pharmacological glucose-lowering intervention, behaviour-changing intervention, placebo or no intervention in people with impaired fasting glucose, impaired glucose tolerance, moderately elevated HbA1c or combinations of these.
Data collection and analysis: Two review authors read all abstracts and full-text articles/records, assessed quality and extracted outcome data independently. One review author extracted data which were checked by a second review author. We resolved discrepancies by consensus or the involvement of a third review author. For meta-analyses we used a random-effects model with investigation of risk ratios (RRs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes, using 95% confidence intervals (CIs) for effect estimates. We carried out trial sequential analyses (TSAs) for all outcomes that could be meta-analysed. We assessed the overall quality of the evidence by using the GRADE instrument.
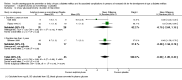
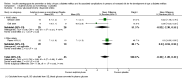
Main results: We included six RCTs with 10,018 participants; 4791 participants with data on allocation to intervention groups were randomised to a second- or third-generation sulphonylurea or a meglitinide analogue as monotherapy and 29 participants were randomised to a second-generation sulphonylurea plus metformin. Three trials investigated a second-generation sulphonylurea, two trials investigated a third-generation sulphonylurea and one trial a meglitinide analogue. A total of 4873 participants with data on allocation to control groups were randomised to a comparator group; 4820 participants were randomised to placebo, 23 to diet and exercise, and 30 participants to metformin monotherapy. One RCT of nateglinide contributed 95% of all participants. The duration of the intervention varied from six months to five years. We judged none of the included trials as at low risk of bias for all 'Risk of bias' domains.All-cause and cardiovascular mortality following sulphonylurea (glimepiride) treatment were rarely observed (very low-quality evidence). The RR for incidence of T2DM comparing glimepiride monotherapy with placebo was 0.75; 95% CI 0.54 to 1.04; P = 0.08; 2 trials; 307 participants; very low-quality evidence. One of the trials reporting on the incidence of T2DM did not define the diagnostic criteria used. The other trial diagnosed T2DM as two consecutive fasting blood glucose values ≥ 6.1 mmol/L. TSA showed that only 4.5% of the diversity-adjusted required information size was accrued so far. No trial reported data on serious adverse events, non-fatal myocardial infarction (MI), non-fatal stroke, congestive heart failure (HF), health-related quality of life or socioeconomic effects.One trial with a follow-up of five years compared a meglitinide analogue (nateglinide) with placebo. A total of 310/4645 (6.7%) participants allocated to nateglinide died compared with 312/4661 (6.7%) participants allocated to placebo (hazard ratio (HR) 1.00; 95% CI 0.85 to 1.17; P = 0.98; moderate-quality evidence). The two main criteria for diagnosing T2DM were a fasting plasma glucose level ≥ 7.0 mmol/L or a 2-hour post challenge glucose ≥ 11.1 mmol/L. T2DM developed in 1674/4645 (36.0%) participants in the nateglinide group and in 1580/4661 (33.9%) in the placebo group (HR 1.07; 95% CI 1.00 to 1.15; P = 0.05; moderate-quality evidence). One or more serious adverse event was reported in 2066/4602 (44.9%) participants allocated to nateglinide compared with 2089/4599 (45.6%) participants allocated to placebo. A total of 126/4645 (2.7%) participants allocated to nateglinide died because of cardiovascular disease compared with 118/4661 (2.5%) participants allocated to placebo (HR 1.07; 95% CI 0.83 to 1.38; P = 0.60; moderate-quality evidence). Comparing participants receiving nateglinide with those receiving placebo for the outcomes MI, non-fatal stroke and HF gave the following event rates: MI 116/4645 (2.5%) versus 122/4661 (2.6%), stroke 100/4645 (2.2%) versus 110/4661 (2.4%) and numbers hospitalised for HF 85/4645 (1.8%) versus 100/4661 (2.1%) - (HR 0.85; 95% CI 0.64 to 1.14; P = 0.27). The quality of the evidence was moderate for all these outcomes. Health-related quality of life or socioeconomic effects were not reported.
Authors' conclusions: There is insufficient evidence to demonstrate whether insulin secretagogues compared mainly with placebo reduce the risk of developing T2DM and its associated complications in people at increased risk for the development of T2DM. Most trials did not investigate patient-important outcomes.
Conflict of interest statement
BH: this review is part of a series of reviews on interventions for the prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus, which is funded by the WHO (Hemmingsen 2016a; Hemmingsen 2016b; Hemmingsen 2016c).
DS: none known.
MIM: none known.
BR: none known.
Figures
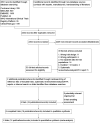
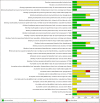
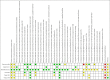
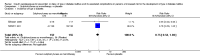










Update of
References
References to studies included in this review
Eriksson 2006 {published data only}
-
- Eriksson JG, Lehtovirta M, Ehrnstrom B, Salmela S, Groop L. Long‐term beneficial effects of glipizide treatment on glucose tolerance in subjects with impaired glucose tolerance. Journal of Internal Medicine 2006;259(6):553‐60. [PUBMED: 16704555] - PubMed
NANSY 2011 {published data only}
-
- Lindblad U, Lindberg G, Mansson NO, Ranstam J, Tyrberg M, Jansson S, et al. Can sulphonylurea addition to lifestyle changes help to delay diabetes development in subjects with impaired fasting glucose? The Nepi ANtidiabetes StudY (NANSY). Diabetes, Obesity & Metabolism 2011; Vol. 13, issue 2:185‐8. [PUBMED: 21199271] - PubMed
-
- Melander A, Lindblad U, Ranstam J. The NEPI Antidiabetes Study (NANSY), a Scandinavian Complement to the DPP Study. Diabetes 1999; Vol. SA351.
-
- Tyrberg M, Melander A, Lovestam‐Adrian M, Lindblad U. Retinopathy in subjects with impaired fasting glucose: the NANSY‐Eye baseline report. Diabetes, Obesity & Metabolism 2008;10(8):646‐51. [PUBMED: 17645554] - PubMed
NAVIGATOR 2010 {published data only}
-
- Novartis clinical result database ‐ NAVIGATOR. www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=3223 (accessed 28 April 2016).
-
- Bethel MA, Chacra AR, Deedwania P, Fulcher GR, Holman RR, Jenssen T, et al. Estimating diabetes risk in patients with impaired glucose tolerance and cardiovascular disease (CVD) or risk factors for CVD. Diabetes 2011;60(0):A357‐8.
-
- Califf RM, Boolell M, Haffner SM, Bethel M, McMurray J, Duggal A, et al. Prevention of diabetes and cardiovascular disease in patients with impaired glucose tolerance: rationale and design of the Nateglinide And Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) Trial. American Heart Journal 2008;156(4):623‐32. [PUBMED: 18946890] - PubMed
-
- Califf RM, Chiang FT, Gaciong Z, Giles TD, Laakso M, Leiter LA, et al. Stroke in people with impaired glucose tolerance. Circulation 2011;124(21).
Osei 2004 {published data only}
-
- Osei K, Rhinesmith S, Gaillard T, Schuster D. Beneficial metabolic effects of chronic glipizide in obese African Americans with impaired glucose tolerance: implications for primary prevention of type 2 diabetes. Metabolism: Clinical and Experimental 2004;53(4):414‐22. [PUBMED: 15045685] - PubMed
Page 1993 {published data only}
-
- Page RC, Harnden KE, Cook JT, Turner RC. Can life‐styles of subjects with impaired glucose tolerance be changed? A feasibility study. Diabetic Medicine 1992;9(6):562‐6. [PUBMED: 1643806] - PubMed
-
- Page RC, Harnden KE, Walravens NK, Onslow C, Sutton P, Levy JC, et al. 'Healthy living' and sulphonylurea therapy have different effects on glucose tolerance and risk factors for vascular disease in subjects with impaired glucose tolerance. The Quarterly Journal of Medicine 1993;86(3):145‐54. [PUBMED: 8483989] - PubMed
Papoz 1978 {published data only}
-
- Eschwege E. French Study Group for Diabetes Epidemiology (A.F.E.D.I.A.). Short term effects (2 years) of oral treatment for diabetes in the borderline impairment of oral glucose tolerance test. Diabetologia 1974;10(363):Abstract 31.
-
- Papoz L, Job D, Eschwege E, Aboulker JP, Cubeau J, Pequignot G, et al. Effect of oral hypoglycaemic drugs on glucose tolerance and insulin secretion in borderline diabetic patients. Diabetologia 1978;15(5):373‐80. [PUBMED: 104897] - PubMed
References to studies excluded from this review
Bavirti 2003 {published data only}
-
- Bavirti S, Tayek JA. Low‐dose oral glyburide reduces glucose production rates in patients with impaired fasting glucose. Metabolism: Clinical and Experimental 2003;52(4):407‐12. [PUBMED: 12701050] - PubMed
Bitzen 1988 {published data only}
-
- Bitzen PO, Melander A, Schersten B, Wahlin‐Boll E. The influence of glipizide on early insulin release and glucose disposal before and after dietary regulation in diabetic patients with different degrees of hyperglycaemia. European Journal of Clinical Pharmacology 1988;35(1):31‐7. [PUBMED: 3065086] - PubMed
Cederholm 1985 {published data only}
-
- Cederholm J. Short‐term treatment of glucose intolerance in middle‐aged subjects by diet, exercise and sulfonylurea. Upsala Journal of Medical Sciences 1985;90(3):229‐42. [PUBMED: 4095819] - PubMed
Cederholm 1986 {published data only}
-
- Cederholm J, Wibell L. The impact of treatment on insulin release and relative peripheral resistance during the oral glucose tolerance test. A study of noninsulin‐dependent diabetes mellitus and glucose intolerance. Diabete & Metabolisme 1986;12(1):10‐5. [PUBMED: 3516748] - PubMed
DIANA 2012 {published data only}
-
- Kataoka Y, Yasuda S, Miyamoto Y, Sase K, Kosuge M, Kimura K, et al. Effects of voglibose and nateglinide on glycemic status and coronary atherosclerosis in early‐stage diabetic patients. Circulation Journal 2012;76(3):712‐20. [PUBMED: 22240597] - PubMed
Gudipaty 2014 {published data only}
-
- NCT00775684. Effect of exenatide, sitagliptin or glimepiride on functional ß‐cell mass. clinicaltrials.gov/ct2/show/NCT00775684 (accessed 3 March 2016).
Hirose 2002 {published data only}
-
- Hirose T, Mizuno R, Yoshimoto T. The effects of nateglinide following oral glucose load in impaired glucose tolerance subjects: rapid insulin stimulation by nateglinide in IGT subjects. Endocrine Journal 2002;49(6):649‐52. [PUBMED: 12625415] - PubMed
Igata 2014 {published data only}
-
- Igata S, Tahara N, Tahara A, Nitta Y, Honda A, Kodama N, et al. Visceral fat metabolic activity is independently associated with coronary artery inflammation in patients with impaired glucose intolerance or type 2 diabetes. Circulation 2014;130.
-
- Kodama N. Effects of pioglitazone versus glimepiride on abdominal fat glucose metabolism in patients with impaired glucose tolerance and/or type 2 diabetes mellitus. Circulation 2012;126(21).
-
- Kodama N, Tahara N, Tahara A, Honda A, Nitta Y, Mizoguchi M, et al. Effects of pioglitazone on visceral fat metabolic activity in impaired glucose tolerance or type 2 diabetes mellitus. Journal of Clinical Endocrinology and Metabolism 2013;98(11):4438‐45. [PUBMED: 24030946] - PubMed
-
- Mizoguchi M, Tahara N, Tahara A, Nitta Y, Kodama N, Oba T, et al. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator‐controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC. Cardiovascular Imaging 2011;4(10):1110‐8. [PUBMED: 21999871] - PubMed
-
- NCT00722631. Anti‐inflammatory effects of pioglitazone. clinicaltrials.gov/ct2/show/NCT00722631 (accessed 22 April 2016).
Inoue 1997 {published data only}
-
- Inoue I, Takahashi K, Noji S, Awata T, Negishi K, Katayama S. Acarbose controls postprandial hyperproinsulinemia in non‐insulin dependent diabetes mellitus. Diabetes Research and Clinical Practice 1997;36(3):143‐51. [PUBMED: 9237780] - PubMed
Johanson 2005 {published data only}
-
- Johanson EH, Jansson PA, Gustafson B, Sandqvist M, Taskinen MR, Smith U, et al. No acute effect of nateglinide on postprandial lipid and lipoprotein responses in subjects at risk for type 2 diabetes. Diabetes/Metabolism Research and Reviews 2005;21(4):376‐81. [PUBMED: 15724236] - PubMed
Katahira 2005 {published data only}
-
- Katahira H, Ishida H. [Effects of mitiglinide in treatment of impaired glucose tolerance]. Nihon Rinsho. Japanese Journal of Clinical Medicine 2005;63 Suppl 2:444‐50. [PUBMED: 15779420] - PubMed
Lindblad 2001 {published data only}
-
- Lindblad U, Lindwall K, Sjostrand A, Ranstam J, Melander A. The NEPI antidiabetes study (NANSY). 1: short‐term dose‐effect relations of glimepiride in subjects with impaired fasting glucose. Diabetes, Obesity & Metabolism 2001;3(6):443‐51. [PUBMED: 11903417] - PubMed
Major‐Pedersen 2008 {published data only}
-
- Major‐Pedersen A, Ihlemann N, Hermann TS, Christiansen B, Kveiborg B, Dominguez H, et al. Effects of acute and chronic attenuation of postprandial hyperglycemia on postglucose‐load endothelial function in insulin resistant individuals: is stimulation of first phase insulin secretion beneficial for the endothelial function?. Hormone and Metabolic Research 2008;40(9):607‐13. [PUBMED: 18792871] - PubMed
-
- NCT00259168. Insulin resistance and vessel function after meals: does early intervention make a difference?. clinicaltrials.gov/ct2/show/NCT00259168?term=NCT00259168&rank=1 (accessed 22 April 2016).
NCT00744965 {published data only}
-
- NCT00744965. Treatment of mild gestational diabetes with glyburide versus placebo. clinicaltrials.gov/ct2/show/NCT00744965 (accessed 17 May 2016).
NCT01563120 {published data only}
-
- NCT01563120. A comparison between two oral hypoglycemics ‐ metformin and glybenclamide for the treatment of gestational diabetes mellitus. clinicaltrials.gov/show/NCT01563120 (accessed 17 March 2016).
Pontiroli 1991 {published data only}
-
- Pontiroli AE, Perfetti MG, Pozza G. Acute effect of glipizide on glucose tolerance in obesity and diabetes mellitus (NIDDM). European Journal of Clinical Pharmacology 1991;40(1):23‐6. [PUBMED: 2060541] - PubMed
-
- Pontiroli AE, Perfetti MG, Pozza G. Improvement of glucose tolerance by minimal doses of glipizide in obese subjects with different degrees of glucose intolerance. Hormone and Metabolic Research. Supplement Series 1992;26:32‐4. [PUBMED: 1490690] - PubMed
Ratzmann 1981 {published data only}
-
- Ratzmann KP, Witt S, Schulz B, Jahr D, Heinke P. [Effect of glibenclamide therapy on carbohydrate and lipid metabolism and insulin secretion in patients with glucose tolerance disorders : a 5‐year study] [Einfluss einer Glibenclamid‐Therapie auf Kohlenhydrat‐ und Fettstoffwechsel sowie Insulinsekretion bei Personen mit gestörter Glukosetorleranz: Verlaufsbeobachtungen bis zu 5 Jahren]. Zeitschrift fur die Gesamte Innere Medizin und ihre Grenzgebiete 1981;36(23):913‐7. [PUBMED: 6805141] - PubMed
Ratzmann 1983 {published data only}
-
- Ratzmann KP, Witt S, Schulz B. The effect of long‐term glibenclamide treatment on glucose tolerance, insulin secretion and serum lipids in subjects with impaired glucose tolerance. Diabète & Métabolisme 1983;9(2):87‐93. [PUBMED: 6413265] - PubMed
Saloranta 2002 {published data only}
-
- Saloranta C, Guitard C, Pecher E, Pablos‐Velasco P, Lahti K, Brunel P, et al. Nateglinide improves early insulin secretion and controls postprandial glucose excursions in a prediabetic population. Diabetes Care 2002;25(12):2141‐6. [PUBMED: 12453951] - PubMed
Schmoelzer 2006 {published data only}
The Fasting Hyperglycaemia Study 1997a {published data only}
-
- Dyson PA, Hammersley MS, Morris RJ, Holman RR, Turner RC. The Fasting Hyperglycaemia Study: II. Randomized controlled trial of reinforced healthy‐living advice in subjects with increased but not diabetic fasting plasma glucose. Metabolism: Clinical and Experimental 1997;46(12 Suppl 1):50‐5. [PUBMED: 9439560] - PubMed
-
- Hammersley MS, Meyer LC, Morris RJ, Manley SE, Turner RC, Holman RR. The Fasting Hyperglycaemia Study: I. Subject identification and recruitment for a non‐insulin‐dependent diabetes prevention trial. Metabolism: Clinical and Experimental 1997;46(12 Suppl 1):44‐9. [PUBMED: 9439559] - PubMed
-
- Karunakaran S, Hammersley MS, Morris RJ, Turner RC, Holman RR. The Fasting Hyperglycaemia Study: III. Randomized controlled trial of sulfonylurea therapy in subjects with increased but not diabetic fasting plasma glucose. Metabolism: Clinical and Experimental 1997;46(12 Suppl 1):56‐60. [PUBMED: 9439561] - PubMed
Additional references
Abdul‐Ghani 2006
-
- Abdul‐Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55(5):1430‐5. [PUBMED: 16644701] - PubMed
ACT NOW 2011
-
- DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. New England Journal of Medicine 2011;364(12):1104‐15. [PUBMED: 21428766] - PubMed
ADA 1997
-
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20(7):1183‐97. - PubMed
ADA 2003
-
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S5‐20. - PubMed
ADA 2008
-
- American Diabetes Association. Standards of medical care in diabetes ‐ 2008. Diabetes Care 2008;31(Suppl 1):S12‐54. [PUBMED: 18165335] - PubMed
ADA 2010
ADA 2015
-
- American Diabetes Association. Standards of medical care in diabetes‐2015. Diabetes Care 2015;38(Suppl 1):S1‐93. [PUBMED: 24357209] - PubMed
ADOPT 2006
-
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. New England Journal of Medicine 2006;355(23):2427‐43. - PubMed
Anderson 2005
-
- Anderson DC Jr. Pharmacologic prevention or delay of type 2 diabetes mellitus. Annals of Pharmacotherapy 2005;39(1):102‐9. [PUBMED: 15562143] - PubMed
Bedford 1982
-
- Keen H, Jarrett RJ, McCartney P. The ten‐year follow‐up of the Bedford survey (1962‐1972): glucose tolerance and diabetes. Diabetologia 1982;22(2):73‐8. [PUBMED: 7060852] - PubMed
Bell 2013
-
- Bell ML, McKenzie JE. Designing psycho‐oncology randomised trials and cluster randomised trials: variance components and intra‐cluster correlation of commonly used psychosocial measures. Psycho‐oncology 2013;22:1738‐47. - PubMed
Beller 2013
Bhardwaj 2010
-
- Bhardwaj A, Agarwal V, Phung OJ, Baker WL, Tongbram V, Coleman CI. Can oral hypoglycemic agents restore normoglycemia in individuals with insulin resistance?. Diabetes. Conference: 70th Scientific Sessions of the American Diabetes Association Orlando, FL United States. 2010; Vol. 0, issue 0.
Black 2007
Blickle 2006
-
- Blickle JF. Meglitinide analogues: a review of clinical data focused on recent trials. Diabetes & Metabolism 2006;32(2):113‐20. [PUBMED: 16735959] - PubMed
Botnia Study 1996
-
- Groop L, Forsblom C, Lehtovirta M, Tuomi T, Karanko S, Nissen M, et al. Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex‐specific parental effects. Diabetes 1996;45(11):1585‐93. [PUBMED: 8866565] - PubMed
Boutron 2014
-
- Boutron I, Altman DG, Hopewell S, Vera‐Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32:4120‐6. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] - PubMed
CDC 2015
-
- Centers for Disease Control and Prevention. 2014 National Diabetes Statistics Report. www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html (accessed 15 October 2015).
Cheng 2006
-
- Cheng C, Kushner H, Falkner BE. The utility of fasting glucose for detection of prediabetes. Metabolism: Clinical and Experimental 2006;55(4):434‐8. [PUBMED: 16546472] - PubMed
ClinicalTrials.gov
-
- clinicaltrials.gov/ct2/results?term=prediabetes+drugs&Search=Search (accessed 12 February 2016).
Corbett 2014
-
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5:79‐85. - PubMed
Deeks 2003
-
- Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non‐randomised intervention studies. Health Technology Assessment (Winchester, England) 2003;7(27):iii‐x, 1‐173. [PUBMED: 14499048] - PubMed
DeFronzo 1989
-
- DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non‐insulin‐dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism: Clinical and Experimental 1989;38(4):387‐95. [PUBMED: 2657323] - PubMed
Diabetes Prevention Program 2002
Diabetes Prevention Program FU 2009
-
- Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. 10‐year follow‐up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374(9702):1677‐86. [DOI: 10.1016/S0140-6736(09)61457-4; PUBMED: 19878986] - DOI - PMC - PubMed
diabetes.co.uk 2016a
-
- Diabetes.co.uk. Convert whole blood results to plasma readings. www.diabetes.co.uk/whole‐blood‐readings‐to‐plasma‐converter.html (assessed 22 August 2016).
diabetes.co.uk 2016b
-
- Diabetes.co.uk. Blood sugar converter. www.diabetes.co.uk/blood‐sugar‐converter.html (assessed 22 August 2016).
DREAM 2008
-
- DREAM Trial Investigators, Dagenais GR, Gerstein HC, Holman R, Budaj A, Escalante A, et al. Effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care 2008;31(5):1007‐14. [DOI: 10.2337/dc07-1868%3C/body%3E%3C/html%3E] - DOI - PubMed
Drugs.com 2016a
-
- Drugs.com. Glimepiride. www.drugs.com/pro/glimepiride.html (accessed 5 October 2016).
Drugs.com 2016b
-
- Drugs.com. Gliclazide 80 mg tablets. www.drugs.com/uk/gliclazide‐80‐mg‐tablets‐leaflet.html (accessed 5 October 2016).
Dunkley 2014
-
- Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta‐analysis. Diabetes Care 2014;37(4):922‐33. [PUBMED: 24652723] - PubMed
Egbewale 2014
Finnish Diabetes Prevention Study Group 2001
-
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne‐Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001;344(18):1343‐50. [PUBMED: 11333990] - PubMed
Gosmanov 2014
-
- Gosmanov AR, Wan J. Low positive predictive value of hemoglobin A1c for diagnosis of prediabetes in clinical practice. American Journal of the Medical Sciences 2014;348(3):191‐4. [PUBMED: 24556928] - PubMed
GRADEproGDT 2015 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEproGDT. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Harrower 2000
-
- Harrower AD. Comparative tolerability of sulphonylureas in diabetes mellitus. Drug Safety 2000;22(4):313‐20. [PUBMED: 10789825] - PubMed
Hemmingsen 2016a
-
- Hemmingsen B, Krogh J, Metzendorf MI, Richter B. Dipeptidyl‐peptidase (DPP)‐4 inhibitors or glucagon‐like peptide (GLP)‐1 analogues for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012204] - DOI - PMC - PubMed
Hemmingsen 2016b
-
- Hemmingsen B, Krogh J, Metzendorf MI, Richter B. Sodium‐glucose cotransporter (SGLT) 2 inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at risk for the development of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD012106.pub2] - DOI - PMC - PubMed
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. - PubMed
Higgins 2003
Higgins 2009
Higgins 2010
Higgins 2011a
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
Hopper 2011
-
- Hopper I, Billah B, Skiba M, Krum H. Prevention of diabetes and reduction in major cardiovascular events in studies of subjects with prediabetes: meta‐analysis of randomised controlled clinical trials. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18(6):813‐23. [PUBMED: 21878448] - PubMed
Hróbjartsson 2013
-
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. - PMC - PubMed
ICH 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice //1997 CFR & ICH Guidelines. PA 19063‐2043. USA: Barnett International/PAREXEL, 1997.
IDF 2013
-
- International Diabetes Federation. IDF Diabetes Atlas. 6th Edition. Brussels: International Diabetes Federation, 2013.
IEC 2009
Jensen 2002
-
- Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE. Beta‐cell function is a major contributor to oral glucose tolerance in high‐risk relatives of four ethnic groups in the U.S. Diabetes 2002;51(7):2170‐8. [PUBMED: 12086947] - PubMed
Kirkham 2010
Lan 1983
-
- Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659‐63.
Liberati 2009
Lundh 2012
Mathieu 2009
-
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977‐84. - PubMed
McCall 2001
-
- McCall AL. Clinical review of glimepiride. Expert Opinion on Pharmacotherapy 2001;2(4):699‐713. [PUBMED: 11336617] - PubMed
Meader 2014
Meigs 2002
-
- Meigs JB, Nathan DM, D'Agostino RB Sr, Wilson PW. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care 2002;25(10):1845‐50. [PUBMED: 12351489] - PubMed
Morris 2013
-
- Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ, et al. Progression rates from HbA1c 6.0‐6.4% and other prediabetes definitions to type 2 diabetes: a meta‐analysis. Diabetologia 2013;56(7):1489‐93. [PUBMED: 23584433] - PubMed
NDDG 1979
-
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes 1979;28(12):1039‐57. [PUBMED: 510803] - PubMed
Pantalone 2012
-
- Pantalone KM, Kattan MW, Yu C, Wells BJ, Arrigain S, Jain A, et al. Increase in overall mortality risk in patients with type 2 diabetes receiving glipizide, glyburide or glimepiride monotherapy versus metformin: a retrospective analysis. Diabetes, Obesity & Metabolism 2012;14(9):803‐9. [PUBMED: 22486923] - PubMed
Phillippe 2013
-
- Phillippe HM, Wargo KA. Mitiglinide for type 2 diabetes treatment. Expert Opinion on Pharmacotherapy 2013;14(15):2133‐44. [PUBMED: 23992284] - PubMed
Phung 2012
-
- Phung OJ, Baker WL, Tongbram V, Bhardwaj A, Coleman CI. Oral antidiabetic drugs and regression from prediabetes to normoglycemia: a meta‐analysis. Annals of Pharmacotherapy 2012;46(4):469‐76. [PUBMED: 22474136] - PubMed
Pogue 1997
-
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93; discussion 661‐6. [PUBMED: 9408720] - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
-
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. - PubMed
Riley 2013
-
- Riley RD, Kauser I, Bland M, Thijs L, Staessen JA, Wang J, et al. Meta‐analysis of randomised trials with a continuous outcome according to baseline imbalance and availability of individual participant data. Statistics in Medicine 2013;32(16):2747‐66. - PubMed
Roumie 2012
Sartor 1980
-
- Sartor G, Schersten B, Carlstrom S, Melander A, Norden A, Persson G. Ten‐year follow‐up of subjects with impaired glucose tolerance: prevention of diabetes by tolbutamide and diet regulation. Diabetes 1980;29(1):41‐9. [PUBMED: 7380107] - PubMed
Schramm 2011
-
- Schramm TK, Gislason GH, Vaag A, Rasmussen JN, Folke F, Hansen ML, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. European Heart Journal 2011;32(15):1900‐8. [PUBMED: 21471135] - PubMed
Scott 2012
-
- Scott LJ. Repaglinide: a review of its use in type 2 diabetes mellitus. Drugs 2012;72(2):249‐72. [PUBMED: 22268393] - PubMed
Selvin 2011
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. - PubMed
UGDP 1976
-
- The University Group Diabetes Program. A study of the effects of hypoglycemic agents on vascular complications in patients with adult‐onset diabetes. VI. Supplementary report on nonfatal events in patients treated with tolbutamide. Diabetes 1976;25:1129‐53. - PubMed
UKPDS 33 1998
-
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352(9131):837‐53. [PUBMED: 9742976] - PubMed
UKPDS 34 1998
-
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352(9131):854‐65. [PUBMED: 9742977] - PubMed
Van de Laar 2006
-
- Laar FA, Lucassen PL, Akkermans RP, Lisdonk EH, Grauw WJ. Alpha‐glucosidase inhibitors for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database of Systematic Reviews 2006;4:CD005061. [PUBMED: 17054235] - PubMed
Viera 2011
-
- Viera AJ. Predisease: when does it make sense?. Epidemiologic Reviews 2011;33:122‐34. - PubMed
Vist 2008
-
- Vist GE, Bryant D, Somerville L, Birminghem T, Oxman AD. Outcomes of patients who participate in randomized controlled trials compared to similar patients receiving similar interventions who do not participate. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.MR000009.pub4] - DOI - PMC - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] - PubMed
WHO 1998
-
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15(7):539‐53. - PubMed
WHO 1999
-
- World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1. Diagnosis and classification of diabetes mellitus. WHO, 1999.
WHO/IDF 2006
-
- World Health Organization/ International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. www.idf.org/webdata/docs/WHO_IDF_definition_diagnosis_of_diabetes.pdf (accessed 10 October 2015).
Wong 2006a
Wong 2006b
Wood 2008
References to other published versions of this review
Hemmingsen 2016c
-
- Hemmingsen B, Sonne DP, Metzendorf MI, Richter B. Insulin secretagogues for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD012151] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

