Synthesis and pre-clinical studies of new amino-acid ester thiazolide prodrugs
- PMID: 27750149
- PMCID: PMC7125651
- DOI: 10.1016/j.ejmech.2016.09.080
Synthesis and pre-clinical studies of new amino-acid ester thiazolide prodrugs
Abstract
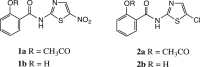
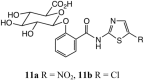
Thiazolides are polypharmacology agents with at least three mechanisms of action against a broad spectrum of parasites, bacteria and viruses. In respiratory viruses they inhibit the replication of orthomyxoviridae and paramyxoviridae at a post-translational level. Nitazoxanide 1a, the prototype thiazolide, was originally developed as an antiparasitic agent and later repurposed for the treatment of viral respiratory infections. The second generation thiazolides following nitazoxanide, such as the 5-chloro analogue RM-5038 2a, are also broad-spectrum antiviral agents as we have reported. Both 1a and its effective circulating metabolite, tizoxanide 1b, are 5-nitrothiazole derivatives, while RM-5038 2a and its de-acetyl derivative RM-4848 2b are the corresponding 5-chloro derivatives. Recently 1a has completed phase II-III clinical trials in the United States, Canada, Australia and New Zealand in a total of 2865 adults and adolescents of at least 12 months of age with viral acute respiratory illness. Since its biodisposition is primarily seen in the gastro-intestinal tract, its efficacy in systemic viral diseases requires relatively high oral doses. The chemical synthesis of new derivatives with a better systemic absorption was therefore urgently needed. In order to improve their systemic absorption, new amino-ester prodrug derivatives of 1b and RM4848 2b were prepared and tested for their animal pharmacology, pharmacokinetics and toxicology. RM-5061 8a in rats showed 7-fold higher blood concentration compared to 1a: absolute bioavailability increased from 3 to 20%, with a good safety profile in animal safety pharmacology and toxicology.
Keywords: Antiviral agents; Biodisposition; Chemical synthesis; Oral absorption; Pharmacology; Prodrugs; Stability; Toxicology.
Copyright © 2016 Elsevier Masson SAS. All rights reserved.
Figures



Similar articles
-
Nitazoxanide: a first-in-class broad-spectrum antiviral agent.Antiviral Res. 2014 Oct;110:94-103. doi: 10.1016/j.antiviral.2014.07.014. Epub 2014 Aug 7. Antiviral Res. 2014. PMID: 25108173 Free PMC article. Review.
-
The susceptibility of circulating human influenza viruses to tizoxanide, the active metabolite of nitazoxanide.Antiviral Res. 2017 Nov;147:142-148. doi: 10.1016/j.antiviral.2017.10.002. Epub 2017 Oct 3. Antiviral Res. 2017. PMID: 28986103
-
Nitazoxanide inhibits paramyxovirus replication by targeting the Fusion protein folding: role of glycoprotein-specific thiol oxidoreductase ERp57.Sci Rep. 2018 Jul 11;8(1):10425. doi: 10.1038/s41598-018-28172-9. Sci Rep. 2018. PMID: 29992955 Free PMC article.
-
5'-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter.Pharm Res. 1998 Aug;15(8):1154-9. doi: 10.1023/a:1011919319810. Pharm Res. 1998. PMID: 9706043
-
Thiazolides: a new class of antiviral drugs.Expert Opin Drug Metab Toxicol. 2009 Jun;5(6):667-74. doi: 10.1517/17425250902988487. Expert Opin Drug Metab Toxicol. 2009. PMID: 19442032 Review.
Cited by
-
Influenza antivirals currently in late-phase clinical trial.Influenza Other Respir Viruses. 2017 May;11(3):240-246. doi: 10.1111/irv.12446. Epub 2017 Feb 28. Influenza Other Respir Viruses. 2017. PMID: 28146320 Free PMC article. Review.
-
Advances in respiratory virus therapeutics - A meeting report from the 6th isirv Antiviral Group conference.Antiviral Res. 2019 Jul;167:45-67. doi: 10.1016/j.antiviral.2019.04.006. Epub 2019 Apr 8. Antiviral Res. 2019. PMID: 30974127 Free PMC article. Review.
-
Amixicile: A Concept Therapeutic for Treatment of Chronic Anaerobic Infections.Br J Gastroenterol. 2020 Feb;2(1):138-142. doi: 10.31488/bjg.1000108. Br J Gastroenterol. 2020. PMID: 37346897 Free PMC article.
-
Prevention and treatment of respiratory viral infections: Presentations on antivirals, traditional therapies and host-directed interventions at the 5th ISIRV Antiviral Group conference.Antiviral Res. 2018 Jan;149:118-142. doi: 10.1016/j.antiviral.2017.11.013. Epub 2017 Nov 21. Antiviral Res. 2018. PMID: 29162476 Free PMC article.
-
Therapeutic potential of salicylamide derivatives for combating viral infections.Med Res Rev. 2023 Jul;43(4):897-931. doi: 10.1002/med.21940. Epub 2023 Mar 10. Med Res Rev. 2023. PMID: 36905090 Free PMC article. Review.
References
-
- Rossignol J.-F., Cavier R. 2-Benzamido-5-Nitrothiazoles. Chem. Abstr. 1975;83:28216n.
-
- Rossignol J.-F., Maisonneuve H. Nitazoxanide in the treatment of Taenia saginata and Hymenolepis nana. Am. J. Trop. Med. Hyg. 1984;33:511–512. - PubMed
-
- Doumbo O., Rossignol J.-F., Pichard E., Traore H., Dembele M., Diakite M., Traore F., Diallo D. Nitazoxanide in the treatment of cryptosporidial diahorrea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa. Am. J. Trop. Med. Hyg. 1997;56:637–639. - PubMed
-
- Rossignol J.-F. Nitazoxanide in the treatment of acquired immune deficiency syndrome-related cryptosporidiosis: results of the United States compassionate programme in 365 patients. Aliment. Pharmacol. Ther. 2006;24:887–894. - PubMed
-
- Fox L.M., Saravolatz M.D. Nitazoxanide: a new thiazolide antiparasitic agent. Clin. Infect. Dis. 2005;40:1173–1180. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

