The digestive tract as the origin of systemic inflammation
- PMID: 27751165
- PMCID: PMC5067918
- DOI: 10.1186/s13054-016-1458-3
The digestive tract as the origin of systemic inflammation
Abstract
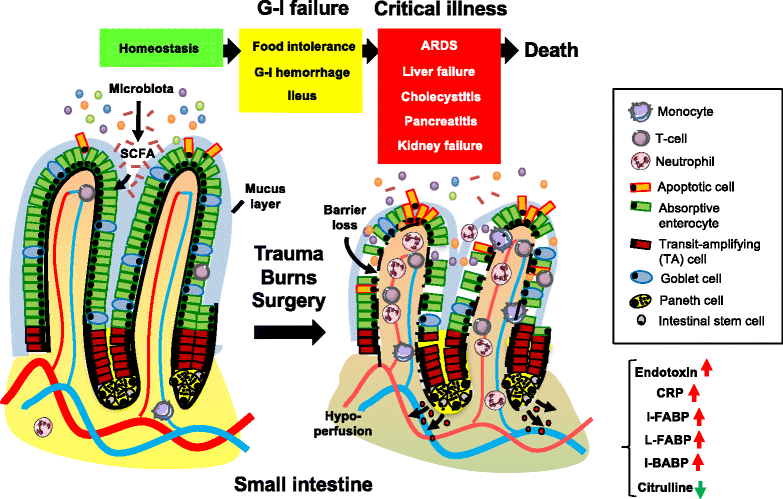
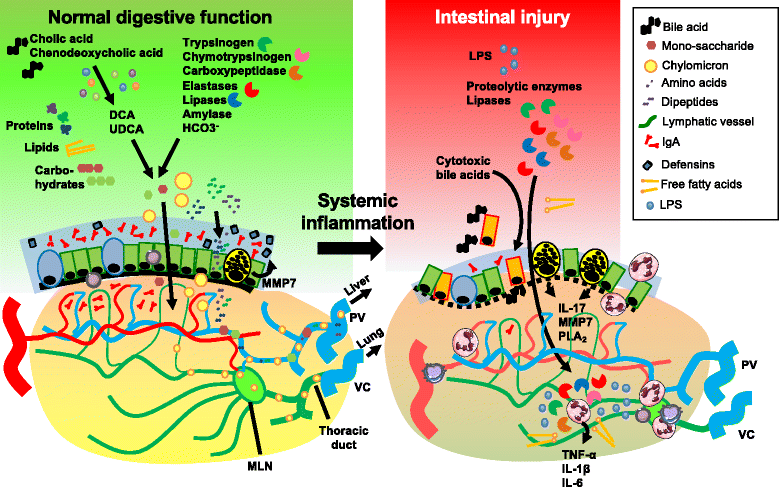
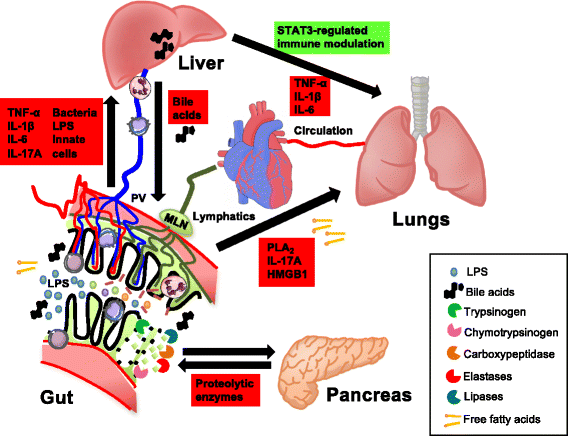
Failure of gut homeostasis is an important factor in the pathogenesis and progression of systemic inflammation, which can culminate in multiple organ failure and fatality. Pathogenic events in critically ill patients include mesenteric hypoperfusion, dysregulation of gut motility, and failure of the gut barrier with resultant translocation of luminal substrates. This is followed by the exacerbation of local and systemic immune responses. All these events can contribute to pathogenic crosstalk between the gut, circulating cells, and other organs like the liver, pancreas, and lungs. Here we review recent insights into the identity of the cellular and biochemical players from the gut that have key roles in the pathogenic turn of events in these organ systems that derange the systemic inflammatory homeostasis. In particular, we discuss the dangers from within the gastrointestinal tract, including metabolic products from the liver (bile acids), digestive enzymes produced by the pancreas, and inflammatory components of the mesenteric lymph.
Keywords: Acute inflammation; Gastrointestinal failure; Gut-liver crosstalk; Pancreatitis.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

