Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis
- PMID: 27751177
- PMCID: PMC5067912
- DOI: 10.1186/s13054-016-1491-2
Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis
Abstract
Background: The dysbiosis of intestinal microbiota plays an important role in the development of gut-derived infections, making it a potential therapeutic target against multiple organ dysfunction syndrome (MODS) after sepsis. However, the effectiveness of fecal microbiota transplantation (FMT) in treating this disease has been rarely investigated.
Methods: Two male patients, a 65-year-old and an 84-year-old, were initially diagnosed with cerebellar hemorrhage and cerebral infarction, respectively, after admission. During the course of hospitalization, both patients developed MODS, septic shock, and severe watery diarrhea. Demographic and clinical data were collected. Intestinal dysbiosis was confirmed by 16S rDNA-based molecular analysis of microbiota composition in fecal samples from the two patients. The two patients each received a single nasogastric infusion of sterile-filtered, pathogen-free feces from a healthy donor. Fecal samples were collected every two days post infusion to monitor changes in microbiota composition in response to treatment.
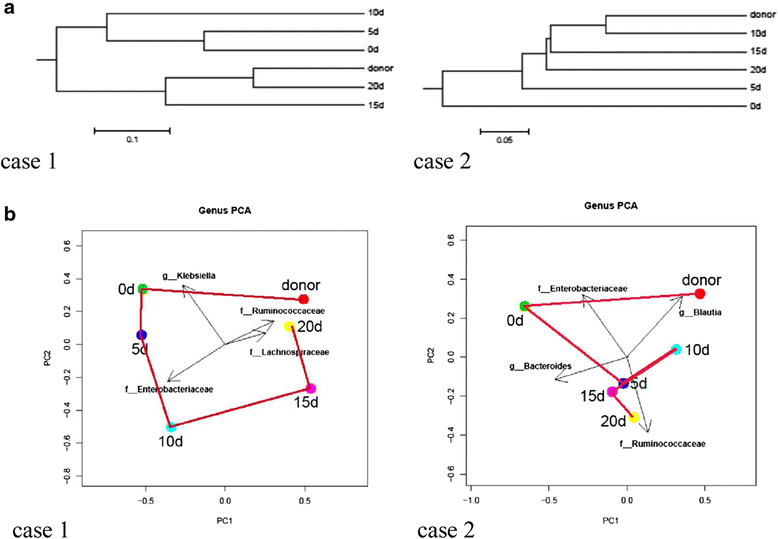
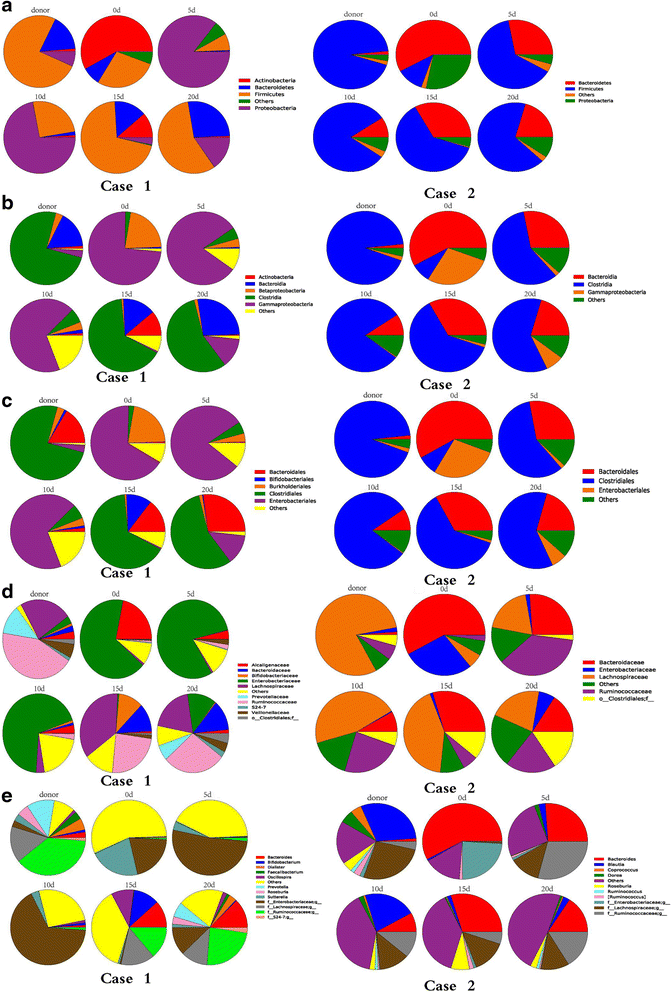
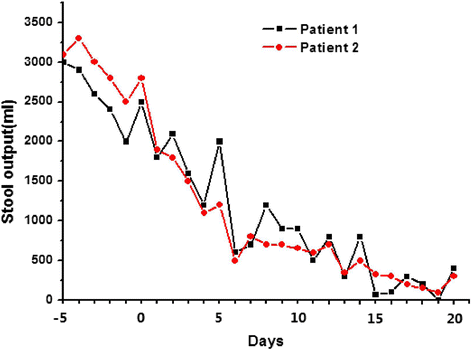
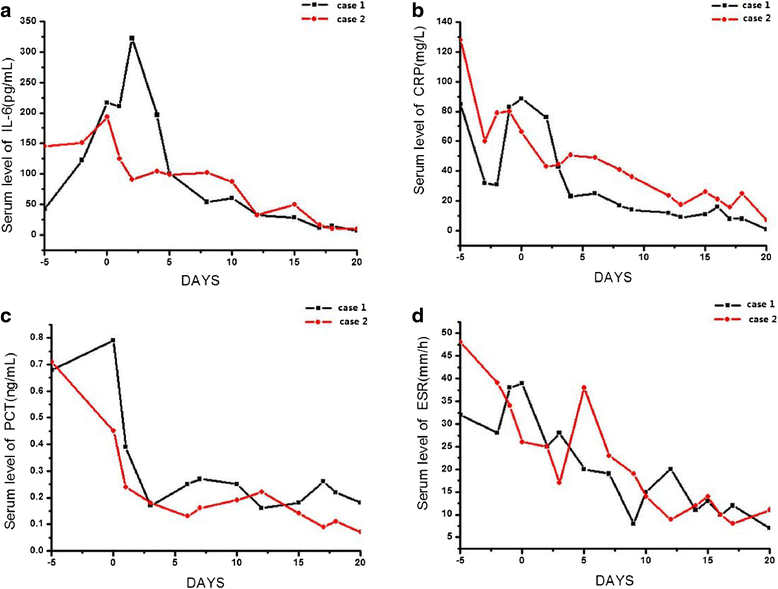
Results: Following FMT, MODS and severe diarrhea were alleviated in both patients. Their stool output and body temperature markedly declined and normalized. Significant modification of microbiota composition, characterized by a profound increase of commensals in the Firmicutes phylum and depletion of opportunistic organisms in the Proteobacteria phylum, was observed in both patients. Furthermore, we identified a reconstituted bacterial community enriched in Firmicutes and depleted of Proteobacteria that was associated with a decrease in the patients' fecal output and in the levels of plasma inflammation markers.
Conclusions: The outcome of treating two patients with FMT indicates that restoration of the intestinal microbiota barrier can alleviate the infection and modulate the immune response. These findings warrant further investigation of FMT as a putative new therapy for treating microbiota-related diseases such as MODS.
Keywords: Diarrhea; Fecal microbiota transplantation; MODS; Therapeutic efficacy.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

