Activity of Pan-Class I Isoform PI3K/mTOR Inhibitor PF-05212384 in Combination with Crizotinib in Ovarian Cancer Xenografts and PDX
- PMID: 27751351
- PMCID: PMC5067927
- DOI: 10.1016/j.tranon.2016.08.011
Activity of Pan-Class I Isoform PI3K/mTOR Inhibitor PF-05212384 in Combination with Crizotinib in Ovarian Cancer Xenografts and PDX
Abstract
The Phosphatidyl inositol-3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) and c-Met signaling pathways are often deregulated in cancer. The two pathways are interconnected and at least c-Met has been implicated in drug resistance. The aim of the study was to assess in ovarian cancer preclinical models, the efficacy and tolerability of a dual PI3K mTOR inhibitor (PF-05212384 or gedatolisib) and a c-Met inhibitor (crizotinib) either as single agents or in combination. In vitro, both PF-05212384 and crizotinib showed a concentration dependent activity in the two ovarian cancer cell lines. The combination of the two did not result in synergistic activity. A subline resistant to gedatolisib was obtained and showed an increased expression of MDR-1 gene. In vivo results show that crizotinib alone did not display any activity in all the tumors investigated, while PF-05212384 alone had some marginal activity. The combination of the two resulted in all the experiments superior to single agents with a good tolerability. Considering that crizotinib did not show activity in the models used, the results indicate that crizotinib is able to potentiate the activity of PF-05212384. Although the activity of the combination was not striking in these three models of ovarian cancer, due to the good tolerability of the combination, the results would suggest the possibility to combine the two drugs in settings in which gedatolisib or crizotinib alone have already some significant activity.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



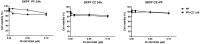




Similar articles
-
Functional Assessments of Gynecologic Cancer Models Highlight Differences Between Single-Node Inhibitors of the PI3K/AKT/mTOR Pathway and a Pan-PI3K/mTOR Inhibitor, Gedatolisib.Cancers (Basel). 2024 Oct 17;16(20):3520. doi: 10.3390/cancers16203520. Cancers (Basel). 2024. PMID: 39456616 Free PMC article.
-
Resistance to dual blockade of the kinases PI3K and mTOR in KRAS-mutant colorectal cancer models results in combined sensitivity to inhibition of the receptor tyrosine kinase EGFR.Sci Signal. 2014 Nov 11;7(351):ra107. doi: 10.1126/scisignal.2005516. Sci Signal. 2014. PMID: 25389372
-
A randomized phase II non-comparative study of PF-04691502 and gedatolisib (PF-05212384) in patients with recurrent endometrial cancer.Gynecol Oncol. 2016 Jul;142(1):62-69. doi: 10.1016/j.ygyno.2016.04.019. Epub 2016 Apr 24. Gynecol Oncol. 2016. PMID: 27103175 Clinical Trial.
-
Targeting the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway: an emerging treatment strategy for squamous cell lung carcinoma.Cancer Treat Rev. 2014 Sep;40(8):980-9. doi: 10.1016/j.ctrv.2014.06.006. Epub 2014 Jul 3. Cancer Treat Rev. 2014. PMID: 25037117 Review.
-
Crizotinib, a small-molecule dual inhibitor of the c-Met and ALK receptor tyrosine kinases.Curr Opin Investig Drugs. 2010 Dec;11(12):1477-90. Curr Opin Investig Drugs. 2010. PMID: 21154129 Review.
Cited by
-
Targeting PI3K/Akt/mTOR in AML: Rationale and Clinical Evidence.J Clin Med. 2020 Sep 11;9(9):2934. doi: 10.3390/jcm9092934. J Clin Med. 2020. PMID: 32932888 Free PMC article. Review.
-
The Application of Patient-Derived Xenograft Models in Gynecologic Cancers.J Cancer. 2020 Jul 11;11(18):5478-5489. doi: 10.7150/jca.46145. eCollection 2020. J Cancer. 2020. PMID: 32742495 Free PMC article. Review.
-
c-Met expression and activity in urogenital cancers - novel aspects of signal transduction and medical implications.Cell Commun Signal. 2017 Feb 17;15(1):10. doi: 10.1186/s12964-017-0165-2. Cell Commun Signal. 2017. PMID: 28212658 Free PMC article. Review.
-
Evaluation of the dual mTOR/PI3K inhibitors Gedatolisib (PF-05212384) and PF-04691502 against ovarian cancer xenograft models.Sci Rep. 2019 Dec 10;9(1):18742. doi: 10.1038/s41598-019-55096-9. Sci Rep. 2019. PMID: 31822716 Free PMC article.
-
Repurposing Osimertinib and Gedatolisib for Glioblastoma Treatment: Evidence of Synergistic Effects in an In Vitro Phenotypic Study.Pharmaceuticals (Basel). 2024 Dec 3;17(12):1623. doi: 10.3390/ph17121623. Pharmaceuticals (Basel). 2024. PMID: 39770465 Free PMC article.
References
-
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. - PubMed
-
- Fry MJ. Structure, regulation and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1994;1226:237–268. - PubMed
-
- Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. - PubMed
-
- Djordjevic S, Driscoll PC. Structural insight into substrate specificity and regulatory mechanisms of phosphoinositide 3-kinases. Trends Biochem Sci. 2002;27:426–432. - PubMed
-
- Foster FM, Traer CJ, Abraham SM, Fry MJ. The phosphoinositide (PI) 3-kinase family. J Cell Sci. 2003;116:3037–3040. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

