Hippocampal to basal forebrain transport of Mn2+ is impaired by deletion of KLC1, a subunit of the conventional kinesin microtubule-based motor
- PMID: 27751944
- PMCID: PMC5457905
- DOI: 10.1016/j.neuroimage.2016.09.035
Hippocampal to basal forebrain transport of Mn2+ is impaired by deletion of KLC1, a subunit of the conventional kinesin microtubule-based motor
Abstract
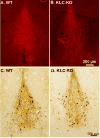
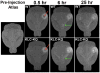
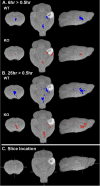
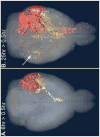
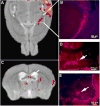
Microtubule-based motors carry cargo back and forth between the synaptic region and the cell body. Defects in axonal transport result in peripheral neuropathies, some of which are caused by mutations in KIF5A, a gene encoding one of the heavy chain isoforms of conventional kinesin-1. Some mutations in KIF5A also cause severe central nervous system defects in humans. While transport dynamics in the peripheral nervous system have been well characterized experimentally, transport in the central nervous system is less experimentally accessible and until now not well described. Here we apply manganese-enhanced magnetic resonance (MEMRI) to study transport dynamics within the central nervous system, focusing on the hippocampal-forebrain circuit, and comparing kinesin-1 light chain 1 knock-out (KLC-KO) mice with age-matched wild-type littermates. We injected Mn2+ into CA3 of the posterior hippocampus and imaged axonal transport in vivo by capturing whole-brain 3D magnetic resonance images (MRI) in living mice at discrete time-points after injection. Precise placement of the injection site was monitored in both MR images and in histologic sections. Mn2+-induced intensity progressed along fiber tracts (fimbria and fornix) in both genotypes to the medial septal nuclei (MSN), correlating in location with the traditional histologic tract tracer, rhodamine dextran. Pairwise statistical parametric mapping (SPM) comparing intensities at successive time-points within genotype revealed Mn2+-enhanced MR signal as it proceeded from the injection site into the forebrain, the expected projection from CA3. By region of interest (ROI) analysis of the MSN, wide variation between individuals in each genotype was found. Despite this statistically significant intensity increases in the MSN at 6h post-injection was found in both genotypes, albeit less so in the KLC-KO. While the average accumulation at 6h was less in the KLC-KO, the difference between genotypes did not reach significance. Projections of SPM T-maps for each genotype onto the same grayscale image revealed differences in the anatomical location of significant voxels. Although KLC-KO mice had smaller brains than wild-type, the gross anatomy was normal with no apparent loss of septal cholinergic neurons. Hence anatomy alone does not explain the differences in SPM maps. We conclude that kinesin-1 defects may have only a minor effect on the rate and distribution of transported Mn2+ within the living brain. This impairment is less than expected for this abundant microtubule-based motor, yet such defects could still be functionally significant, resulting in cognitive/emotional dysfunction due to decreased replenishments of synaptic vesicles or mitochondria during synaptic activity. This study demonstrates the power of MEMRI to observe and measure vesicular transport dynamics in the central nervous system that may result from or lead to brain pathology.
Keywords: Axonal transport; Hippocampal-forebrain circuit; Kinesin light chain-1; Kinesin-1; Manganese-enhanced magnetic resonance imaging (MEMRI); Statistical parametric mapping.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Evolution of brain-wide activity in the awake behaving mouse after acute fear by longitudinal manganese-enhanced MRI.Neuroimage. 2020 Nov 15;222:116975. doi: 10.1016/j.neuroimage.2020.116975. Epub 2020 May 28. Neuroimage. 2020. PMID: 32474079 Free PMC article.
-
Studying Axonal Transport in the Brain by Manganese-Enhanced Magnetic Resonance Imaging (MEMRI).Methods Mol Biol. 2022;2431:111-142. doi: 10.1007/978-1-0716-1990-2_6. Methods Mol Biol. 2022. PMID: 35412274
-
Harnessing axonal transport to map reward circuitry: Differing brain-wide projections from medial forebrain domains.bioRxiv [Preprint]. 2023 Sep 10:2023.09.10.557059. doi: 10.1101/2023.09.10.557059. bioRxiv. 2023. Update in: Front Cell Dev Biol. 2023 Nov 30;11:1278831. doi: 10.3389/fcell.2023.1278831. PMID: 38328063 Free PMC article. Updated. Preprint.
-
Longitudinal manganese-enhanced magnetic resonance imaging of neural projections and activity.NMR Biomed. 2022 Jun;35(6):e4675. doi: 10.1002/nbm.4675. Epub 2022 Mar 6. NMR Biomed. 2022. PMID: 35253280 Free PMC article. Review.
-
In vivo imaging of axonal transport using MRI: aging and Alzheimer's disease.Eur J Nucl Med Mol Imaging. 2008 Mar;35 Suppl 1:S89-92. doi: 10.1007/s00259-007-0707-8. Eur J Nucl Med Mol Imaging. 2008. PMID: 18204931 Review.
Cited by
-
Herpes Simplex Virus, Alzheimer's Disease and a Possible Role for Rab GTPases.Front Cell Dev Biol. 2019 Aug 7;7:134. doi: 10.3389/fcell.2019.00134. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31448273 Free PMC article.
-
Epac activation inhibits IL-6-induced cardiac myocyte dysfunction.J Physiol Sci. 2018 Jan;68(1):77-87. doi: 10.1007/s12576-016-0509-5. Epub 2016 Dec 19. J Physiol Sci. 2018. PMID: 27995459 Free PMC article.
-
Harnessing axonal transport to map reward circuitry: Differing brain-wide projections from medial prefrontal cortical domains.Front Cell Dev Biol. 2023 Nov 30;11:1278831. doi: 10.3389/fcell.2023.1278831. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38099294 Free PMC article.
-
Reconfiguration of brain-wide neural activity after early life adversity.bioRxiv [Preprint]. 2025 May 29:2023.09.10.557058. doi: 10.1101/2023.09.10.557058. bioRxiv. 2025. PMID: 38328213 Free PMC article. Preprint.
-
Evolution of brain-wide activity in the awake behaving mouse after acute fear by longitudinal manganese-enhanced MRI.Neuroimage. 2020 Nov 15;222:116975. doi: 10.1016/j.neuroimage.2020.116975. Epub 2020 May 28. Neuroimage. 2020. PMID: 32474079 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

