Gender and developmental specific N-glycomes of the porcine parasite Oesophagostomum dentatum
- PMID: 27751954
- PMCID: PMC5201199
- DOI: 10.1016/j.bbagen.2016.10.011
Gender and developmental specific N-glycomes of the porcine parasite Oesophagostomum dentatum
Abstract
Background: The porcine nodule worm Oesophagostomum dentatum is a strongylid class V nematode rather closely related to the model organism Caenorhabditis elegans. However, in contrast to the non-parasitic C. elegans, the parasitic O. dentatum is an obligate sexual organism, which makes both a gender and developmental glycomic comparison possible.
Methods: Different enzymatic and chemical methods were used to release N-glycans from male and female O. dentatum as well as from L3 and L4 larvae. Glycans were analysed by MALDI-TOF MS after either 2D-HPLC (normal then reversed phase) or fused core RP-HPLC.
Results: Whereas the L3 N-glycome was simpler and more dominated by phosphorylcholine-modified structures, the male and female worms express a wide range of core fucosylated N-glycans with up to three fucose residues. Seemingly, simple methylated paucimannosidic structures can be considered 'male', while methylation of fucosylated glycans was more pronounced in females. On the other hand, while many of the fucosylated paucimannosidic glycans are identical with examples from other nematode species, but simpler than the tetrafucosylated glycans of C. elegans, there is a wide range of phosphorylcholine-modified glycans with extended HexNAc2-4PC2-4 motifs not observed in our previous studies on other nematodes.
Conclusion: The interspecies tendency of class V nematodes to share most, but not all, N-glycans applies also to O. dentatum; furthermore, we establish, for the first time in a parasitic nematode, that glycomes vary upon development and sexual differentiation.
General significance: Unusual methylated, core fucosylated and phosphorylcholine-containing N-glycans vary between stages and genders in a parasitic nematode.
Keywords: Glycomics; HPLC; Mass spectrometry; Nematoda; Strongylida.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures


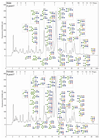

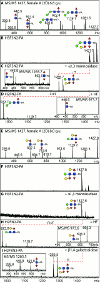



Similar articles
-
Increasing Complexity of the N-Glycome During Caenorhabditis Development.Mol Cell Proteomics. 2023 Mar;22(3):100505. doi: 10.1016/j.mcpro.2023.100505. Epub 2023 Jan 28. Mol Cell Proteomics. 2023. PMID: 36717059 Free PMC article.
-
N-glycans of the porcine nematode parasite Ascaris suum are modified with phosphorylcholine and core fucose residues.FEBS J. 2007 Feb;274(3):714-26. doi: 10.1111/j.1742-4658.2006.05615.x. Epub 2006 Dec 20. FEBS J. 2007. PMID: 17181538 Free PMC article.
-
The parasitic nematode Oesophagostomum dentatum synthesizes unusual glycosaminoglycan-like O-glycans.Glycobiology. 2018 Jul 1;28(7):474-481. doi: 10.1093/glycob/cwy045. Glycobiology. 2018. PMID: 29757381 Free PMC article.
-
Glycans of parasitic nematodes - from glycomes to novel diagnostic tools and vaccines.Carbohydr Res. 2025 Apr;550:109407. doi: 10.1016/j.carres.2025.109407. Epub 2025 Jan 22. Carbohydr Res. 2025. PMID: 39879943 Review.
-
Cracking the nodule worm code advances knowledge of parasite biology and biotechnology to tackle major diseases of livestock.Biotechnol Adv. 2015 Nov 1;33(6 Pt 1):980-91. doi: 10.1016/j.biotechadv.2015.05.004. Epub 2015 May 27. Biotechnol Adv. 2015. PMID: 26026709 Free PMC article. Review.
Cited by
-
New insights into the N-glycomes of Dictyostelium species.BBA Adv. 2025 Jan 16;7:100142. doi: 10.1016/j.bbadva.2025.100142. eCollection 2025. BBA Adv. 2025. PMID: 39911813 Free PMC article.
-
Increasing Complexity of the N-Glycome During Caenorhabditis Development.Mol Cell Proteomics. 2023 Mar;22(3):100505. doi: 10.1016/j.mcpro.2023.100505. Epub 2023 Jan 28. Mol Cell Proteomics. 2023. PMID: 36717059 Free PMC article.
-
Let's talk about sexes: sex-related N-glycosylation in ecologically important invertebrates.Glycoconj J. 2020 Feb;37(1):41-46. doi: 10.1007/s10719-019-09866-2. Epub 2019 Apr 2. Glycoconj J. 2020. PMID: 30941612 Review.
-
Method comparison for N-glycan profiling: Towards the standardization of glycoanalytical technologies for cell line analysis.PLoS One. 2019 Oct 7;14(10):e0223270. doi: 10.1371/journal.pone.0223270. eCollection 2019. PLoS One. 2019. PMID: 31589631 Free PMC article.
-
MALDI-TOF mass spectrometry as a diagnostic tool in human and veterinary helminthology: a systematic review.Parasit Vectors. 2019 May 17;12(1):245. doi: 10.1186/s13071-019-3493-9. Parasit Vectors. 2019. PMID: 31101120 Free PMC article.
References
-
- McSorley HJ, Hewitson JP, Maizels RM. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. 2013;43:301–310. - PubMed
-
- Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis. 2014;14:1150–1162. - PubMed
-
- Polderman AM, Krepel HP, Baeta S, Blotkamp J, Gigase P. Oesophagostomiasis, a common infection of man in northern Togo and Ghana. Am J Trop Med Hyg. 1991;44:336–344. - PubMed
-
- Polderman AM, Blotkamp J. Oesophagostomum infections in humans. Parasitol Today. 1995;11:451–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

