Peripheral administration of lactate produces antidepressant-like effects
- PMID: 27752076
- PMCID: PMC5794893
- DOI: 10.1038/mp.2016.179
Peripheral administration of lactate produces antidepressant-like effects
Erratum in
-
Peripheral administration of lactate produces antidepressant-like effects.Mol Psychiatry. 2018 Feb;23(2):488. doi: 10.1038/mp.2016.237. Epub 2016 Dec 6. Mol Psychiatry. 2018. PMID: 27922608 Free PMC article.
Abstract
In addition to its role as metabolic substrate that can sustain neuronal function and viability, emerging evidence supports a role for l-lactate as an intercellular signaling molecule involved in synaptic plasticity. Clinical and basic research studies have shown that major depression and chronic stress are associated with alterations in structural and functional plasticity. These findings led us to investigate the role of l-lactate as a potential novel antidepressant. Here we show that peripheral administration of l-lactate produces antidepressant-like effects in different animal models of depression that respond to acute and chronic antidepressant treatment. The antidepressant-like effects of l-lactate are associated with increases in hippocampal lactate levels and with changes in the expression of target genes involved in serotonin receptor trafficking, astrocyte functions, neurogenesis, nitric oxide synthesis and cAMP signaling. Further elucidation of the mechanisms underlying the antidepressant effects of l-lactate may help to identify novel therapeutic targets for the treatment of depression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

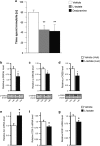

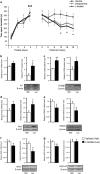
Similar articles
-
Role of adult hippocampal neurogenesis in the antidepressant actions of lactate.Mol Psychiatry. 2021 Nov;26(11):6723-6735. doi: 10.1038/s41380-021-01122-0. Epub 2021 May 14. Mol Psychiatry. 2021. PMID: 33990772 Free PMC article.
-
Role of SIRT1-mediated synaptic plasticity and neurogenesis: Sex-differences in antidepressant-like efficacy of catalpol.Phytomedicine. 2024 Dec;135:156120. doi: 10.1016/j.phymed.2024.156120. Epub 2024 Oct 4. Phytomedicine. 2024. PMID: 39395323
-
Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases.Neuropsychopharmacology. 2019 May;44(6):1152-1162. doi: 10.1038/s41386-019-0313-z. Epub 2019 Jan 8. Neuropsychopharmacology. 2019. PMID: 30647450 Free PMC article.
-
Signaling pathways involved in antidepressant-induced cell proliferation and synaptic plasticity.Curr Pharm Des. 2014;20(23):3776-94. doi: 10.2174/13816128113196660736. Curr Pharm Des. 2014. PMID: 24180397 Review.
-
Neural plasticity to stress and antidepressant treatment.Biol Psychiatry. 1999 Nov 1;46(9):1181-91. doi: 10.1016/s0006-3223(99)00177-8. Biol Psychiatry. 1999. PMID: 10560024 Review.
Cited by
-
Major Depressive Disorder and Gut Microbiota: Role of Physical Exercise.Int J Mol Sci. 2023 Nov 28;24(23):16870. doi: 10.3390/ijms242316870. Int J Mol Sci. 2023. PMID: 38069198 Free PMC article. Review.
-
Lactate: a prospective target for therapeutic intervention in psychiatric disease.Neural Regen Res. 2024 Jul 1;19(7):1473-1479. doi: 10.4103/1673-5374.387969. Epub 2023 Nov 8. Neural Regen Res. 2024. PMID: 38051889 Free PMC article.
-
Effects of Monoamines and Antidepressants on Astrocyte Physiology: Implications for Monoamine Hypothesis of Depression.J Exp Neurosci. 2018 Jul 23;12:1179069518789149. doi: 10.1177/1179069518789149. eCollection 2018. J Exp Neurosci. 2018. PMID: 30046253 Free PMC article. Review.
-
Plasma metabolites changes in male heroin addicts during acute and protracted withdrawal.Aging (Albany NY). 2021 Jul 19;13(14):18669-18688. doi: 10.18632/aging.203311. Epub 2021 Jul 19. Aging (Albany NY). 2021. PMID: 34282053 Free PMC article.
-
Role of Histone Lactylation in Neurological Disorders.Int J Mol Sci. 2025 Aug 18;26(16):7949. doi: 10.3390/ijms26167949. Int J Mol Sci. 2025. PMID: 40869269 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

