DIXDC1 contributes to psychiatric susceptibility by regulating dendritic spine and glutamatergic synapse density via GSK3 and Wnt/β-catenin signaling
- PMID: 27752079
- PMCID: PMC5395363
- DOI: 10.1038/mp.2016.184
DIXDC1 contributes to psychiatric susceptibility by regulating dendritic spine and glutamatergic synapse density via GSK3 and Wnt/β-catenin signaling
Abstract
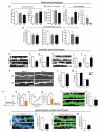
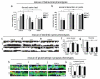
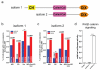
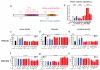
Mice lacking DIX domain containing-1 (DIXDC1), an intracellular Wnt/β-catenin signal pathway protein, have abnormal measures of anxiety, depression and social behavior. Pyramidal neurons in these animals' brains have reduced dendritic spines and glutamatergic synapses. Treatment with lithium or a glycogen synthase kinase-3 (GSK3) inhibitor corrects behavioral and neurodevelopmental phenotypes in these animals. Analysis of DIXDC1 in over 9000 cases of autism, bipolar disorder and schizophrenia reveals higher rates of rare inherited sequence-disrupting single-nucleotide variants (SNVs) in these individuals compared with psychiatrically unaffected controls. Many of these SNVs alter Wnt/β-catenin signaling activity of the neurally predominant DIXDC1 isoform; a subset that hyperactivate this pathway cause dominant neurodevelopmental effects. We propose that rare missense SNVs in DIXDC1 contribute to psychiatric pathogenesis by reducing spine and glutamatergic synapse density downstream of GSK3 in the Wnt/β-catenin pathway.
Figures

References
-
- Shiomi K, Uchida H, Keino-Masu K, Masu M. Ccd1, a novel protein with a DIX domain, is a positive regulator in the Wnt signaling during zebrafish neural patterning. Curr Biol. 2003 Jan 8;13(1):73–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

