Massive release of extracellular vesicles from cancer cells after photodynamic treatment or chemotherapy
- PMID: 27752092
- PMCID: PMC5067517
- DOI: 10.1038/srep35376
Massive release of extracellular vesicles from cancer cells after photodynamic treatment or chemotherapy
Abstract
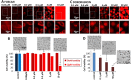
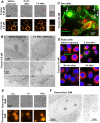
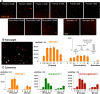
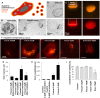
Photodynamic therapy is an emerging cancer treatment that is particularly adapted for localized malignant tumor. The phototherapeutic agent is generally injected in the bloodstream and circulates in the whole organism as a chemotherapeutic agent, but needs light triggering to induce localized therapeutic effects. We found that one of the responses of in vitro and in vivo cancer cells to photodynamic therapy was a massive production and emission of extracellular vesicles (EVs): only 1 hour after the photo-activation, thousands of vesicles per cell were emitted in the extracellular medium. A similar effect has been found after treatment with Doxorubicin (chemotherapy), but far less EVs were produced, even 24 hours after the treatment. Furthermore, we found that the released EVs could transfer extracellular membrane components, drugs and even large intracellular objects to naive target cells. In vivo, photodynamic treatment and chemotherapy increased the levels of circulating EVs several fold, confirming the vast induction of cancer cell vesiculation triggered by anti-cancer therapies.
Figures

Similar articles
-
Cetuximab treatment alters the content of extracellular vesicles released from tumor cells.Nanomedicine (Lond). 2016 Apr;11(8):881-90. doi: 10.2217/nnm-2015-0009. Epub 2016 Mar 16. Nanomedicine (Lond). 2016. PMID: 27021928
-
PML-RARa modulates the vascular signature of extracellular vesicles released by acute promyelocytic leukemia cells.Angiogenesis. 2016 Jan;19(1):25-38. doi: 10.1007/s10456-015-9486-1. Epub 2015 Sep 15. Angiogenesis. 2016. PMID: 26374632
-
Extracellular vesicle-mediated transfer of CLIC1 protein is a novel mechanism for the regulation of glioblastoma growth.Oncotarget. 2015 Oct 13;6(31):31413-27. doi: 10.18632/oncotarget.5105. Oncotarget. 2015. PMID: 26429879 Free PMC article.
-
Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation.Semin Cell Dev Biol. 2017 Jul;67:11-22. doi: 10.1016/j.semcdb.2017.01.003. Epub 2017 Jan 8. Semin Cell Dev Biol. 2017. PMID: 28077296 Review.
-
Tumor-Derived Apoptotic Vesicles: With Death They Do Part.Front Immunol. 2018 May 7;9:957. doi: 10.3389/fimmu.2018.00957. eCollection 2018. Front Immunol. 2018. PMID: 29780392 Free PMC article. Review.
Cited by
-
High frequency acoustic cell stimulation promotes exosome generation regulated by a calcium-dependent mechanism.Commun Biol. 2020 Oct 5;3(1):553. doi: 10.1038/s42003-020-01277-6. Commun Biol. 2020. PMID: 33020585 Free PMC article.
-
Studying Unconventional Secretion of Misfolded Proteins in Cultured Cells and Primary Neurons.Methods Mol Biol. 2022;2473:349-366. doi: 10.1007/978-1-0716-2209-4_22. Methods Mol Biol. 2022. PMID: 35819775
-
Extracellular Vesicles as Mediators and Potential Targets in Combating Cancer Drug Resistance.Molecules. 2025 Jan 23;30(3):498. doi: 10.3390/molecules30030498. Molecules. 2025. PMID: 39942602 Free PMC article. Review.
-
What Is the Role of HLA-I on Cancer Derived Extracellular Vesicles? Defining the Challenges in Characterisation and Potential Uses of This Ligandome.Int J Mol Sci. 2021 Dec 17;22(24):13554. doi: 10.3390/ijms222413554. Int J Mol Sci. 2021. PMID: 34948350 Free PMC article. Review.
-
Engineering of MSC-Derived Exosomes: A Promising Cell-Free Therapy for Osteoarthritis.Membranes (Basel). 2022 Jul 28;12(8):739. doi: 10.3390/membranes12080739. Membranes (Basel). 2022. PMID: 36005656 Free PMC article. Review.
References
-
- Balkwill F., Charles K. A. & Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7, 211–217 (2005). - PubMed
-
- Rivoltini L. et al.. Escape strategies and reasons for failure in the interaction between tumour cells and the immune system: how can we tilt the balance towards immune-mediated cancer control? Expert Opin Biol Ther 5, 463–476 (2005). - PubMed
-
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 5, 263–274 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

