Strategies and Systems-Level Interventions to Combat or Prevent Drug Counterfeiting: A Systematic Review of Evidence Beyond Effectiveness
- PMID: 27752221
- PMCID: PMC5045490
- DOI: 10.1007/s40290-016-0156-4
Strategies and Systems-Level Interventions to Combat or Prevent Drug Counterfeiting: A Systematic Review of Evidence Beyond Effectiveness
Abstract
Background: A recent systematic review suggested that drug registrations and onsite quality inspections may be effective in reducing the prevalence of counterfeit and substandard drugs. However, simply replicating the most effective interventions is problematic, as it denotes implementing the intervention without further adaptation.
Objective: The aim was to systematically review the evidence beyond effectiveness for systems-level interventions to combat or prevent drug counterfeiting.
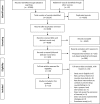
Methods: We conducted an extensive search, including an electronic search of 14 databases. We included studies examining the efficiency, feasibility, reliability, and economic outcomes of the interventions, as well as barriers and facilitators to their implementation. Two reviewers selected eligible studies and abstracted data in duplicate and independently. We synthesized the results narratively, stratified by type of intervention.
Results: Of 10,220 captured citations, 19 met our inclusion criteria. The findings suggest that the following may strengthen regulatory measures (e.g., registration): minimizing drug diversion, enhancing lines of communications, ensuring feedback on drug quality, and promoting strict licensing criteria. There is evidence that onsite quality surveillance and inspection systems may be efficient and cost-effective for preliminary testing of large samples of drugs. Laws and legislation need to be specific to counterfeit drugs, include firm penalties, address online purchasing of drugs, and be complemented by education of judges and lawyers. Public awareness and education should rely on multiple platforms and comprehensive and dedicated content. While product authentication technologies may be efficient and reliable in detecting counterfeit drugs in the supply chain, they require a strong information system infrastructure. As for pharmacovigilance systems, it is critical to tackle the issue of underreporting, to enhance their chances of success.
Conclusion: Several factors are critical to the successful design and implementation of systems-level interventions to combat or prevent drug counterfeiting. Policymakers need to take these into consideration to ensure success of these interventions.
Conflict of interest statement
Compliance with Ethical Standards Funding This work was supported by the Alliance for Health Policy and Systems Research at the WHO, Grant Number 102716. The funding body was not involved in the design of the study, data collection, analysis, interpretation of findings, or in writing the manuscript. Open access was funded by the Alliance for Health Policy and Systems Research. Conflict of interest Racha Fadlallah, Fadi El-Jardali, Farah Annan, Hayat Azzam, and Elie Akl declare that they have no conflict of interest.
Figures
References
-
- Buowari OV. Fake and counterfeit drug: a review. Afrimedic J. 2013;3(2):1–4.
-
- Chika A, Bello SO, Jimoh AO, Umar MT. The menace of fake drugs: consequences, causes and possible solutions. Res J Med Sci. 2006;1(5):257–261.
-
- Lancet. Fighting fake drugs: the role of WHO and pharma. Lancet. 2011;377(9778):1626. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous