Design and engineering of a transmissible antiviral defense
- PMID: 27752283
- PMCID: PMC5062863
- DOI: 10.1186/s13036-016-0033-4
Design and engineering of a transmissible antiviral defense
Abstract
Background: We propose, model, and implement a novel system of population-level intervention against a virus. One context is a treatment against a chronic infection such as HIV. The underlying principle is a form of virus 'wars' in which a benign, transmissible agent is engineered to protect against infection by and spread of a lethal virus. In our specific case, the protective agent consists of two entities, a benign virus and a gene therapy vector mobilized by the benign virus.
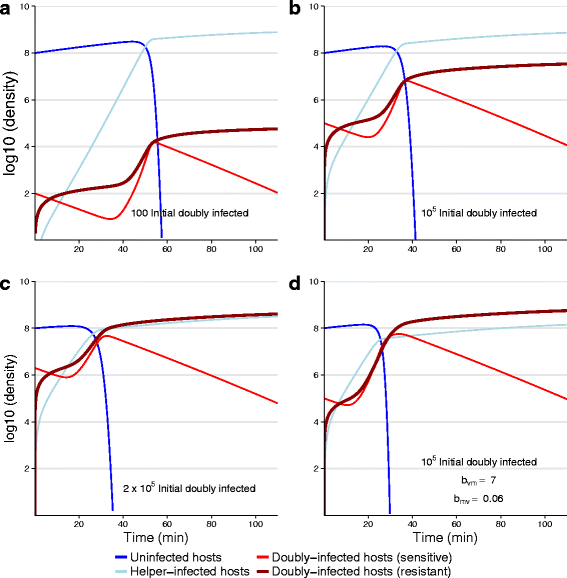
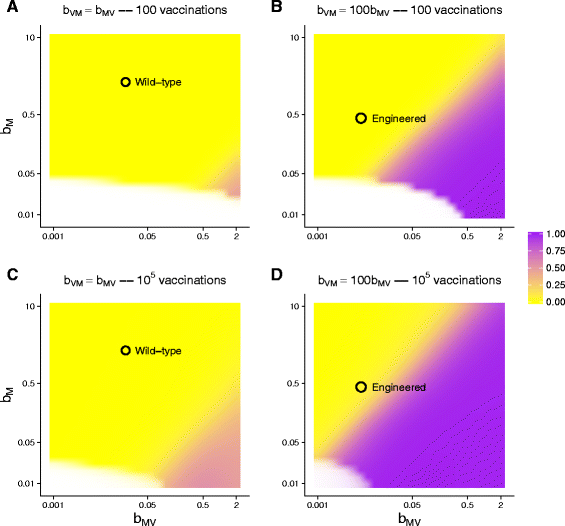
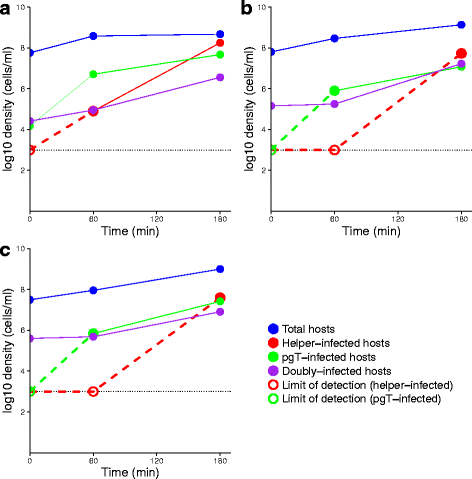
Results: Numerical analysis of a mathematical model identified parameter ranges in which adequate, population-wide protection is achieved. The protective system was implemented and tested using E. coli, bacteriophage M13 and a phagemid vector mobilized by M13 to block infection by the lethal phage T5. Engineering of M13 profoundly improved its dynamical properties for facilitating spread of the gene therapy vector. However, the gene therapy vector converts the host cell to resist T5 too slowly for protection on a time scale appropriate for T5.
Conclusions: Overall, there is a reasonable marriage between the mathematical model and the empirical system, suggesting that such models can be useful guides to the design of such systems even before the models incorporate most of the relevant biological details.
Keywords: Bacteriophage; Genetic engineering; Transmissible vaccine; Virus.
Figures



Similar articles
-
Virus wars: using one virus to block the spread of another.PeerJ. 2016 Jun 29;4:e2166. doi: 10.7717/peerj.2166. eCollection 2016. PeerJ. 2016. PMID: 27413636 Free PMC article.
-
M13 bacteriophage DNA inhibits duck hepatitis B virus during acute infection.Hepatology. 1994 May;19(5):1079-87. Hepatology. 1994. PMID: 8175129
-
Eliminating helper phage from phage display.Nucleic Acids Res. 2006;34(21):e145. doi: 10.1093/nar/gkl772. Epub 2006 Nov 6. Nucleic Acids Res. 2006. PMID: 17088290 Free PMC article.
-
Research Progress of M13 Bacteriophage-Based Biosensors.Nanomaterials (Basel). 2019 Oct 11;9(10):1448. doi: 10.3390/nano9101448. Nanomaterials (Basel). 2019. PMID: 31614669 Free PMC article. Review.
-
Chemical modulation of M13 bacteriophage and its functional opportunities for nanomedicine.Int J Nanomedicine. 2014 Dec 12;9:5825-36. doi: 10.2147/IJN.S73883. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25540583 Free PMC article. Review.
References
-
- Richman DD. Antiviral Drug Resistance. Chichester; New York: Wiley; 1996.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

