Histone modification signature at myeloperoxidase and proteinase 3 in patients with anti-neutrophil cytoplasmic autoantibody-associated vasculitis
- PMID: 27752292
- PMCID: PMC5057507
- DOI: 10.1186/s13148-016-0251-0
Histone modification signature at myeloperoxidase and proteinase 3 in patients with anti-neutrophil cytoplasmic autoantibody-associated vasculitis
Abstract
Background: Anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV) is a systemic autoimmune disease characterized by destructive vascular inflammation. Two prominent ANCA autoantigens are myeloperoxidase (MPO) and proteinase 3 (PR3), and transcription of MPO and PRTN3, the genes encoding the autoantigens, is associated with disease activity. We investigated whether patients with AAV have alterations in histone modifications, particularly those associated with transcriptional activation, at MPO and PRTN3.
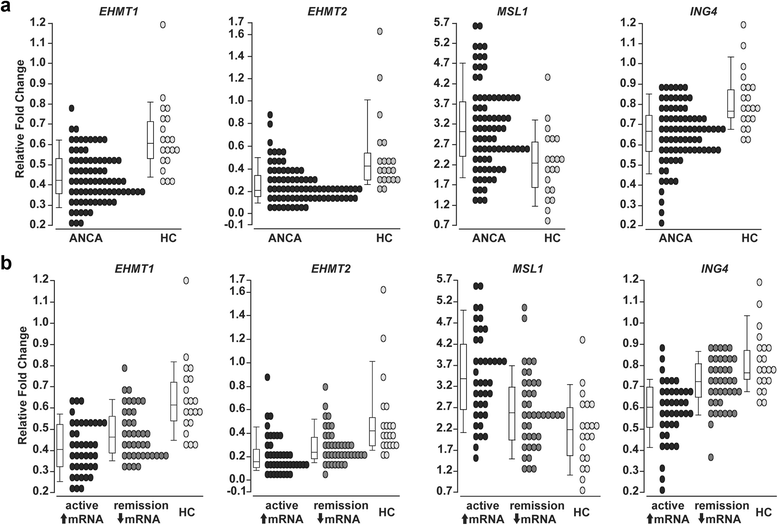
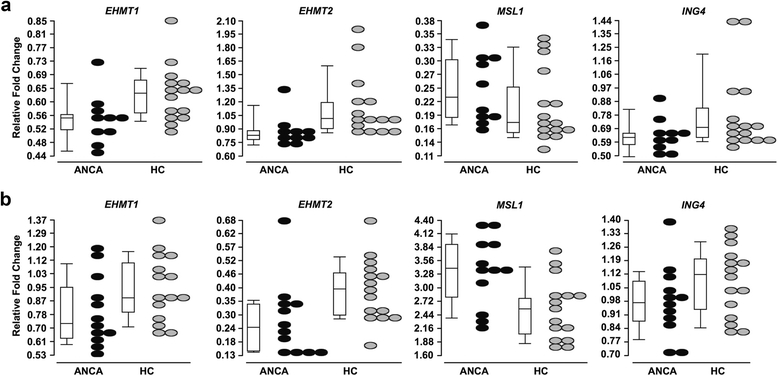
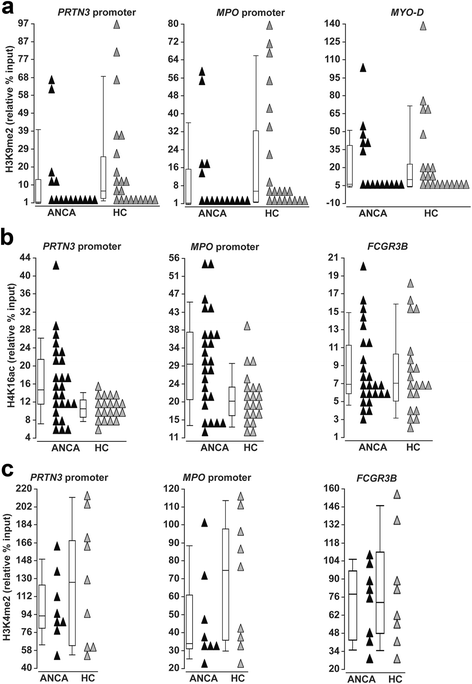
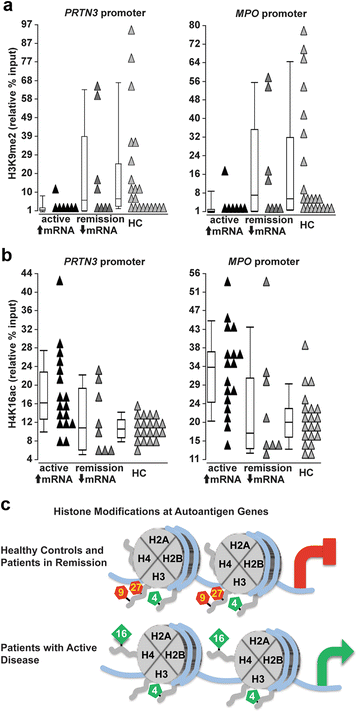
Results: We identified a network of genes regulating histone modifications that were differentially expressed in AAV patients compared to healthy controls. We focused on four genes (EHMT1 and EHMT2, ING4, and MSL1) and found their expression correlated with expression of MPO and PRTN3. Methylation of histone H3K9, catalyzed by EHMT1 and EHMT2 and associated with gene silencing, was most depleted at MPO and PRTN3 in patients with active disease and the highest MPO and PRTN3 expression. Acetylation of histone H4K16, modified by complexes containing ING4 and MSL1 and associated with gene activation, was most enriched at MPO and PRTN3 in patients with active disease and the highest MPO and PRTN3 expression. Methylation at H3K4, a mark of transcriptional activation, was enriched at MPO and PRTN3 in patients and healthy controls.
Conclusions: MPO and PRTN3 in neutrophils of AAV patients with active disease have a distinct pattern of histone modifications, which implicates epigenetic mechanisms in regulating expression of autoantigen genes and suggests that the epigenome may be involved in AAV pathogenesis.
Keywords: ANCA-associated vasculitis; Autoantigens; Epigenetics; Gene expression; Neutrophils.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

