Terminal Dopamine Release Kinetics in the Accumbens Core and Shell Are Distinctly Altered after Withdrawal from Cocaine Self-Administration
- PMID: 27752541
- PMCID: PMC5052666
- DOI: 10.1523/ENEURO.0274-16.2016
Terminal Dopamine Release Kinetics in the Accumbens Core and Shell Are Distinctly Altered after Withdrawal from Cocaine Self-Administration
Abstract
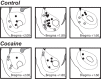
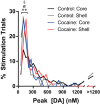
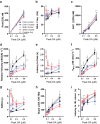
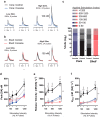
Repeated self-administration of cocaine is associated with impairments in motivated behaviors as well as alterations in both dopamine (DA) release and neural signaling within the nucleus accumbens (NAc). These impairments are present even after several weeks of abstinence from drug taking, suggesting that the self-administration experience induces long-lasting neuroplastic alterations in the mesolimbic DA circuit. To understand these changes at the terminal level, rats were allowed to self-administer either cocaine intravenously (∼1 mg/kg per infusion) or water to a receptacle (control) in 2-h sessions over 14 days, followed by 30 days of enforced abstinence. Fast-scan cyclic voltammetry was used to record real-time DA release in either NAc core or shell after electrical stimulations of the ventral tegmental area (VTA) in freely-moving animals. In controls, the kinetics of DA release in the core and shell strikingly differed, with shell displaying slower release and reuptake rates than core. However, cocaine experience differentially altered these signaling patterns by NAc subregion. In the shell, cocaine rats showed less sensitivity to the dynamic range of applied stimulations than controls. In the core, by contrast, cocaine rats displayed robustly reduced peak DA release given the same stimulation, while also showing slower release and reuptake kinetics. The differential effects of cocaine self-administration on terminal function between core and shell is consistent with a region-specific functional reorganization of the mesolimbic DA system after repeated exposure and may provide an anatomical substrate for altered cognitive function after chronic drug-taking and addiction.
Keywords: Michaelis–Menten; dopamine transporter; drug addiction; plasticity; ventral striatum; voltammetry.
Figures


References
-
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM (2008) Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci 28:8821–8831. 10.1523/JNEUROSCI.2225-08.2008 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
