Nanoscale chirality in metal and semiconductor nanoparticles
- PMID: 27752651
- PMCID: PMC5317218
- DOI: 10.1039/c6cc05613j
Nanoscale chirality in metal and semiconductor nanoparticles
Abstract
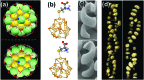
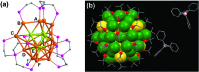
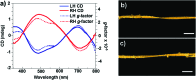
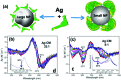
The field of chirality has recently seen a rejuvenation due to the observation of chirality in inorganic nanomaterials. The advancements in understanding the origin of nanoscale chirality and the potential applications of chiroptical nanomaterials in the areas of optics, catalysis and biosensing, among others, have opened up new avenues toward new concepts and design of novel materials. In this article, we review the concept of nanoscale chirality in metal nanoclusters and semiconductor quantum dots, then focus on recent experimental and theoretical advances in chiral metal nanoparticles and plasmonic chirality. Selected examples of potential applications and an outlook on the research on chiral nanomaterials are additionally provided.
Figures












Similar articles
-
Plasmonic Chirality and Circular Dichroism in Bioassembled and Nonbiological Systems: Theoretical Background and Recent Progress.Adv Mater. 2020 Oct;32(41):e1801790. doi: 10.1002/adma.201801790. Epub 2018 Sep 9. Adv Mater. 2020. PMID: 30260543 Review.
-
Inorganic Chiral Hybrid Nanostructures for Tailored Chiroptics and Chirality-Dependent Photocatalysis.Angew Chem Int Ed Engl. 2022 Jun 13;61(24):e202112400. doi: 10.1002/anie.202112400. Epub 2022 Jan 20. Angew Chem Int Ed Engl. 2022. Retraction in: Angew Chem Int Ed Engl. 2022 Sep 5;61(36):e202210734. doi: 10.1002/anie.202210734. PMID: 34936187 Retracted.
-
Tunable Chiroptical Activity in Branched Ag@Au Nanoparticles with Molecular Chirality and Geometric Chirality.Small. 2025 Feb;21(7):e2410021. doi: 10.1002/smll.202410021. Epub 2024 Dec 23. Small. 2025. PMID: 39711280
-
Chiral Plasmonic Hybrid Nanostructures: A Gateway to Advanced Chiroptical Materials.Adv Mater. 2024 Jan;36(3):e2309033. doi: 10.1002/adma.202309033. Epub 2023 Nov 23. Adv Mater. 2024. PMID: 37944554 Review.
-
Multicomponent chiral plasmonic hybrid nanomaterials: recent advances in synthesis and applications.Nanoscale Adv. 2023 Dec 6;6(2):318-336. doi: 10.1039/d3na00808h. eCollection 2024 Jan 16. Nanoscale Adv. 2023. PMID: 38235081 Free PMC article. Review.
Cited by
-
Design principles of chiral carbon nanodots help convey chirality from molecular to nanoscale level.Nat Commun. 2018 Aug 24;9(1):3442. doi: 10.1038/s41467-018-05561-2. Nat Commun. 2018. PMID: 30143608 Free PMC article.
-
Engineering copper plasmonic chirality via ligand-induced dissolution for enantioselective recognition of amino acids.Chem Sci. 2024 Apr 16;15(19):7121-7129. doi: 10.1039/d4sc00477a. eCollection 2024 May 15. Chem Sci. 2024. PMID: 38756802 Free PMC article.
-
Temperature dependent chiroptical response of sigmoidal gold clusters: probing the stability of chiral metal clusters.Chem Sci. 2018 May 25;9(25):5614-5622. doi: 10.1039/c8sc00344k. eCollection 2018 Jul 7. Chem Sci. 2018. PMID: 30061994 Free PMC article.
-
Bringing chiral functionality to in vivo applications of nanomaterials.Light Sci Appl. 2022 May 27;11(1):157. doi: 10.1038/s41377-022-00841-5. Light Sci Appl. 2022. PMID: 35624097 Free PMC article.
-
Reconfigurable homochiral colloidal clusters assembled under orthogonally applied electric and magnetic fields.Proc Natl Acad Sci U S A. 2025 Apr 8;122(14):e2418006122. doi: 10.1073/pnas.2418006122. Epub 2025 Apr 1. Proc Natl Acad Sci U S A. 2025. PMID: 40168128
References
-
- Kondepudi D. K., Durand D. J. Chirality. 2001;13:351–356. - PubMed
-
- Addadi L., Weiner S. Nature. 2001;411:753–755. - PubMed
-
- Watson J. D., Crick F. H. C. Nature. 1953;171:737–738. - PubMed
-
- Levene P. A. Chem. Rev. 1925;2:179–216.
-
- Buschmann H., Thede R., Heller D. Angew. Chem., Int. Ed. 2000;39:4033–4036. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

