Optical tracer size differences allow quantitation of active pumping rate versus Stokes-Einstein diffusion in lymphatic transport
- PMID: 27752703
- PMCID: PMC5067306
- DOI: 10.1117/1.JBO.21.10.100501
Optical tracer size differences allow quantitation of active pumping rate versus Stokes-Einstein diffusion in lymphatic transport
Abstract
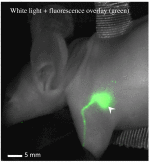
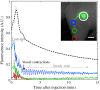
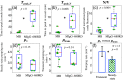
Lymphatic uptake of interstitially administered agents occurs by passive convective–diffusive inflow driven by interstitial concentration and pressure, while the downstream lymphatic transport is facilitated by active propulsive contractions of lymphatic vessel walls. Near-infrared fluorescence imaging in mice was used to measure these central components of lymphatic transport for the first time, using two different-sized molecules––methylene blue (MB) and fluorescence-labeled antibody immunoglobulin G (IgG)-IRDye 680RD. This work confirms the hypothesis that lymphatic passive inflow and active propulsion rates can be separated based upon the relative differences in Stokes–Einstein diffusion coefficient. This coefficient specifically affects the passive-diffusive uptake when the interstitial volume and pressure are constant. Parameters such as mean time-to-peak signal, overall fluorescence signal intensities, and number of active peristaltic pulses, were estimated from temporal imaging data. While the mean time to attain peak signal representative of diffusion-dominated flow in the lymph vessels was 0.6±0.2??min for MB and 8±6??min for IgG, showing a size dependence, the active propulsion rates were 3.4±0.8??pulses/min and 3.3±0.5??pulses/min, respectively, appearing size independent. The propulsion rates for both dyes decreased with clearance from the interstitial injection-site, indicating intrinsic control of the smooth muscles in response to interstitial pressure. This approach to size-comparative agent flow imaging of lymphatic function can enable noninvasive characterization of diseases related to uptake and flow in lymph networks.
Figures

References
-
- Schmid-Schonbein G. W., “Microlymphatics and lymph flow,” Physiol. Rev. 70(4), 987–1028 (1990).PHREA7 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

