Interaction of hemin with quadruplex DNA
- PMID: 27752804
- PMCID: PMC5323342
- DOI: 10.1007/s10867-016-9430-7
Interaction of hemin with quadruplex DNA
Abstract
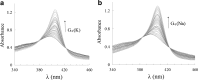
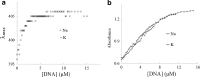
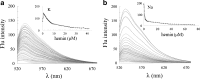
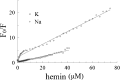
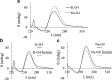
A DNA enzyme with peroxidase activity is a G-quadruplex-based DNAzyme formed by hemin and G-quadruplex DNA. Activity of peroxide DNAzymes can be influenced by the structure of quadruplex DNA. In this investigation, the interaction of hemin with T30695 G-quadruplex DNA is evaluated. Molecular dynamic simulation indicates that the binding mode of hemin to G-quadruplex DNA is end-stacking, which is consistent with absorption spectroscopy. Based on fluorescence spectroscopy, hemin ejects thiazole orange from bases of four-strand DNA. Circular dichroism spectra showed that no alteration occurs in this type of DNA structure. Graphical Abstract Peroxidase DNAzyme is formed by hemin and G-quadruplex DNA.
Keywords: DNAzyme; Hemin; Interaction; Peroxidase; Quadruplex.
Figures

References
-
- Babunageswararao K, Ramakrishna R, Kumar PDK, Sameer S. DNAzymes as novel therapeutics. Int J Pharm. Bio. Sci. 2011;2:161–173.
-
- Golub E, Lu CH, Willner I. Metalloporphyrin/G-quadruplexes: From basic properties to practical applications. J. Porphyrins Phthalocyanines. 2015;19:65–91. doi: 10.1142/S1088424615300025. - DOI
-
- Lee KS, Raymond LD, Schoen B, Raymond GJ, Kett L, Moore RA, Johnson LM, Taubner L, Speare JO, Onwubiko HA, Baron GS, Caughey WS, Caughey B. Hemin interactions and alterations of the subcellular localization of prion protein. J. Biol. Chem. 2007;282:36525–36533. doi: 10.1074/jbc.M705620200. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

