Nanomechanical properties of MscL α helices: A steered molecular dynamics study
- PMID: 27753526
- PMCID: PMC5463901
- DOI: 10.1080/19336950.2016.1249077
Nanomechanical properties of MscL α helices: A steered molecular dynamics study
Abstract
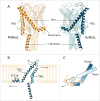
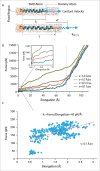
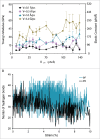
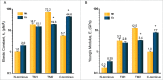
Gating of mechanosensitive (MS) channels is driven by a hierarchical cascade of movements and deformations of transmembrane helices in response to bilayer tension. Determining the intrinsic mechanical properties of the individual transmembrane helices is therefore central to understanding the intricacies of the gating mechanism of MS channels. We used a constant-force steered molecular dynamics (SMD) approach to perform unidirectional pulling tests on all the helices of MscL in M. tuberculosis and E. coli homologs. Using this method, we could overcome the issues encountered with the commonly used constant-velocity SMD simulations, such as low mechanical stability of the helix during stretching and high dependency of the elastic properties on the pulling rate. We estimated Young's moduli of the α-helices of MscL to vary between 0.2 and 12.5 GPa with TM2 helix being the stiffest. We also studied the effect of water on the properties of the pore-lining TM1 helix. In the absence of water, this helix exhibited a much stiffer response. By monitoring the number of hydrogen bonds, it appears that water acts like a 'lubricant' (softener) during TM1 helix elongation. These data shed light on another physical aspect underlying hydrophobic gating of MS channels, in particular MscL.
Keywords: Escherichia coli; Young's modulus; all-atom simulation; constant velocity; mechanosensitive channel; mycobacterium tuberculosis; nanovalve.
Figures


Comment in
-
Channel disassembled: Pick, tweak, and soak parts to soften.Channels (Austin). 2017 May 4;11(3):173-175. doi: 10.1080/19336950.2017.1291213. Epub 2017 Feb 6. Channels (Austin). 2017. PMID: 28166450 Free PMC article. No abstract available.
Similar articles
-
Pulling MscL open via N-terminal and TM1 helices: A computational study towards engineering an MscL nanovalve.PLoS One. 2017 Aug 31;12(8):e0183822. doi: 10.1371/journal.pone.0183822. eCollection 2017. PLoS One. 2017. PMID: 28859093 Free PMC article.
-
A finite element framework for studying the mechanical response of macromolecules: application to the gating of the mechanosensitive channel MscL.Biophys J. 2006 Aug 15;91(4):1248-63. doi: 10.1529/biophysj.106.085985. Epub 2006 May 26. Biophys J. 2006. PMID: 16731564 Free PMC article.
-
The gating mechanism of the bacterial mechanosensitive channel MscL revealed by molecular dynamics simulations: from tension sensing to channel opening.Channels (Austin). 2012 Jul-Aug;6(4):317-31. doi: 10.4161/chan.21895. Channels (Austin). 2012. PMID: 23146938 Free PMC article.
-
Structure and function of the bacterial mechanosensitive channel of large conductance.Protein Sci. 1999 Oct;8(10):1915-21. doi: 10.1110/ps.8.10.1915. Protein Sci. 1999. PMID: 10548036 Free PMC article. Review.
-
Towards an understanding of the structural and functional properties of MscL, a mechanosensitive channel in bacteria.Biol Cell. 1996;87(1-2):1-8. Biol Cell. 1996. PMID: 9004483 Review.
Cited by
-
Channel disassembled: Pick, tweak, and soak parts to soften.Channels (Austin). 2017 May 4;11(3):173-175. doi: 10.1080/19336950.2017.1291213. Epub 2017 Feb 6. Channels (Austin). 2017. PMID: 28166450 Free PMC article. No abstract available.
-
Pocket delipidation induced by membrane tension or modification leads to a structurally analogous mechanosensitive channel state.Structure. 2022 Apr 7;30(4):608-622.e5. doi: 10.1016/j.str.2021.12.004. Epub 2022 Jan 4. Structure. 2022. PMID: 34986323 Free PMC article.
-
Nanomechanical DNA resonators for sensing and structural analysis of DNA-ligand complexes.Nat Commun. 2019 Apr 12;10(1):1690. doi: 10.1038/s41467-019-09612-0. Nat Commun. 2019. PMID: 30979901 Free PMC article.
-
Pulling MscL open via N-terminal and TM1 helices: A computational study towards engineering an MscL nanovalve.PLoS One. 2017 Aug 31;12(8):e0183822. doi: 10.1371/journal.pone.0183822. eCollection 2017. PLoS One. 2017. PMID: 28859093 Free PMC article.
-
Structural Dynamics of the MscL C-terminal Domain.Sci Rep. 2017 Dec 8;7(1):17229. doi: 10.1038/s41598-017-17396-w. Sci Rep. 2017. PMID: 29222414 Free PMC article.
References
-
- Berrier C, Besnard M, Ajouz B, Coulombe A, Ghazi A. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J Membrane Biol 1996; 151:175-87; https://doi.org/10.1007/s002329900068 - DOI - PubMed
-
- Meyer GR, Gullingsrud J, Schulten K, Martinac B. Molecular dynamics study of MscL interactions with a curved lipid bilayer. Biophys J 2006; 91:1630-7; PMID:16751236; https://doi.org/10.1529/biophysj.106.080721 - DOI - PMC - PubMed
-
- Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature 1994; 368:265-8; PMID:7511799; https://doi.org/10.1038/368265a0 - DOI - PubMed
-
- Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature 2009; 459:379-85; PMID:19458714; https://doi.org/10.1038/nature08147 - DOI - PMC - PubMed
-
- Kung C. A possible unifying principle for mechanosensation. Nature 2005; 436:647-54; PMID:16079835; https://doi.org/10.1038/nature03896 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
