Histone chaperone ASF1B promotes human β-cell proliferation via recruitment of histone H3.3
- PMID: 27753532
- PMCID: PMC5176155
- DOI: 10.1080/15384101.2016.1241914
Histone chaperone ASF1B promotes human β-cell proliferation via recruitment of histone H3.3
Abstract
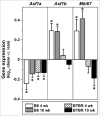
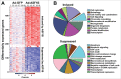
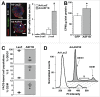
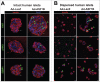
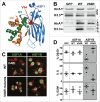
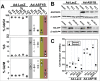
Anti-silencing function 1 (ASF1) is a histone H3-H4 chaperone involved in DNA replication and repair, and transcriptional regulation. Here, we identify ASF1B, the mammalian paralog to ASF1, as a proliferation-inducing histone chaperone in human β-cells. Overexpression of ASF1B led to distinct transcriptional signatures consistent with increased cellular proliferation and reduced cellular death. Using multiple methods of monitoring proliferation and mitotic progression, we show that overexpression of ASF1B is sufficient to induce human β-cell proliferation. Co-expression of histone H3.3 further augmented β-cell proliferation, whereas suppression of endogenous H3.3 attenuated the stimulatory effect of ASF1B. Using the histone binding-deficient mutant of ASF1B (V94R), we show that histone binding to ASF1B is required for the induction of β-cell proliferation. In contrast to H3.3, overexpression of histone H3 variants H3.1 and H3.2 did not have an impact on ASF1B-mediated induction of proliferation. Our findings reveal a novel role of ASF1B in human β-cell replication and show that ASF1B and histone H3.3A synergistically stimulate human β-cell proliferation.
Keywords: ASF1B; cell cycle; histone H3.3; replication-independent histone deposition; β-cell proliferation.
Figures
Comment in
-
ASF1B chaperones histone 3.3 to the β-cell cycle dance.Cell Cycle. 2017 Jan 17;16(2):161-162. doi: 10.1080/15384101.2016.1260989. Epub 2016 Nov 18. Cell Cycle. 2017. PMID: 27860543 Free PMC article. No abstract available.
References
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52:102-10; PMID:12502499; http://dx.doi.org/ 10.2337/diabetes.52.1.102 - DOI - PubMed
-
- Rankin MM, Kushner JA. Adaptive β-cell proliferation is severely restricted with advanced age. Diabetes 2009; 58:1365-72; PMID:19265026; http://dx.doi.org/ 10.2337/db08-1198 - DOI - PMC - PubMed
-
- Salpeter SJ, Khalaileh A, Weinberg-Corem N, Ziv O, Glaser B, Dor Y. Systemic regulation of the age-related decline of pancreatic β-cell replication. Diabetes 2013; 62:2843-8; PMID:23630298; http://dx.doi.org/ 10.2337/db13-0160 - DOI - PMC - PubMed
-
- Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, Butler PC. Adaptive changes in pancreatic β cell fractional area and β cell turnover in human pregnancy. Diabetologia 2010; 53:2167-76; PMID:20523966; http://dx.doi.org/ 10.1007/s00125-010-1809-6 - DOI - PMC - PubMed
-
- Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal β cell mass. J Clin Invest 2004; 114:963-8; PMID:15467835; http://dx.doi.org/ 10.1172/JCI22098 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
