A Randomized, Open-Label Trial to Evaluate Switching to Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide Plus Darunavir in Treatment-Experienced HIV-1-Infected Adults
- PMID: 27753684
- PMCID: PMC5228611
- DOI: 10.1097/QAI.0000000000001193
A Randomized, Open-Label Trial to Evaluate Switching to Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide Plus Darunavir in Treatment-Experienced HIV-1-Infected Adults
Abstract
Background: HIV-infected, treatment-experienced adults with a history of prior resistance and regimen failure can be virologically suppressed but may require multitablet regimens associated with lower adherence and potential resistance development.
Methods: We enrolled HIV-infected, virologically suppressed adults with 2-class to 3-class drug resistance and at least 2 prior regimen failures into this phase 3, open-label, randomized study. The primary endpoint was the percentage of participants with HIV-1 RNA <50 copies per milliliter at week 24 [Food and Drug Administration (FDA) snapshot algorithm].
Results: For 135 participants [elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) plus darunavir (DRV), n = 89; baseline regimen, n = 46], most of whom were taking a median of 5 tablets/d, simplification to E/C/F/TAF plus DRV was noninferior to continuation of baseline regimens at week 24 (plasma HIV-1 RNA <50 copies per milliliter: 96.6% vs. 91.3%, difference 5.3%, 95.001% CI: -3.4% to 17.4%). E/C/F/TAF plus DRV met prespecified criteria for noninferiority and superiority at week 48 for the same outcome. E/C/F/TAF plus DRV was well tolerated and had an improved renal safety profile compared with baseline regimens, with statistically significant differences between groups in quantitative total proteinuria and markers of proximal tubular proteinuria. Compared with baseline regimens, participants who switched to E/C/F/TAF plus DRV reported higher mean treatment satisfaction scale total scores and fewer days with missed doses.
Conclusions: This study demonstrated that regimen simplification from a 5-tablet regimen to the 2-tablet, once-daily combination of E/C/F/TAF plus DRV has durable maintenance of virologic suppression and improvements in specific markers of renal safety. Such a strategy may lead to greater adherence and improved quality of life.
Figures
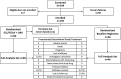
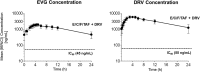



References
-
- Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. - PubMed
-
- Lee LM, Karon JM, Selik R, et al. Survival after AIDS diagnosis in adolescents and adults during the treatment era, United States 1984–1997. JAMA. 2001;285:1308–1315. - PubMed
-
- Valdez H, Chowdhry TK, Asaad R, et al. Changing spectrum of mortality due to human immunodeficiency virus: analysis of 260 deaths during 1995–1999. Clin Infect Dis. 2001;32:1487–1493. - PubMed
-
- Lucas G. Antiretroviral adherence, drug resistance, viral fitness and HIV disease progression: a tangled web is woven. J Antimicrob Chemother. 2005;55:413–416. - PubMed
-
- Taiwo B, Murphy RL, Katlama C. Novel antiretroviral combinations in treatment-experienced patients with HIV infection. Drugs. 2010;70:1629–1642. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

