The Importance of Patient-Specific Factors for Hepatic Drug Response and Toxicity
- PMID: 27754327
- PMCID: PMC5085745
- DOI: 10.3390/ijms17101714
The Importance of Patient-Specific Factors for Hepatic Drug Response and Toxicity
Abstract
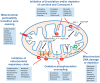
Responses to drugs and pharmacological treatments differ considerably between individuals. Importantly, only 50%-75% of patients have been shown to react adequately to pharmacological interventions, whereas the others experience either a lack of efficacy or suffer from adverse events. The liver is of central importance in the metabolism of most drugs. Because of this exposed status, hepatotoxicity is amongst the most common adverse drug reactions and hepatic liabilities are the most prevalent reason for the termination of development programs of novel drug candidates. In recent years, more and more factors were unveiled that shape hepatic drug responses and thus underlie the observed inter-individual variability. In this review, we provide a comprehensive overview of different principle mechanisms of drug hepatotoxicity and illustrate how patient-specific factors, such as genetic, physiological and environmental factors, can shape drug responses. Furthermore, we highlight other parameters, such as concomitantly prescribed medications or liver diseases and how they modulate drug toxicity, pharmacokinetics and dynamics. Finally, we discuss recent progress in the field of in vitro toxicity models and evaluate their utility in reflecting patient-specific factors to study inter-individual differences in drug response and toxicity, as this understanding is necessary to pave the way for a patient-adjusted medicine.
Keywords: drug-induced liver injury; hepatotoxicity; liver disease; pharmacogenetics.
Conflict of interest statement
The authors are founders and owners of HepaPredict AB.
Figures


References
-
- Frueh F.W., Amur S., Mummaneni P., Epstein R.S., Aubert R.E., DeLuca T.M., Verbrugge R.R., Burckart G.J., Lesko L.J. Pharmacogenomic Biomarker Information in Drug Labels Approved by the United States Food and Drug Administration: Prevalence of Related Drug Use. Pharmacotherapy. 2008;28:992–998. doi: 10.1592/phco.28.8.992. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

