Selectivity Enhancement in Molecularly Imprinted Polymers for Binding of Bisphenol A
- PMID: 27754429
- PMCID: PMC5087485
- DOI: 10.3390/s16101697
Selectivity Enhancement in Molecularly Imprinted Polymers for Binding of Bisphenol A
Abstract
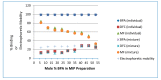
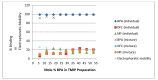
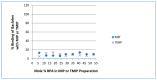
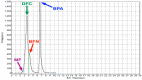
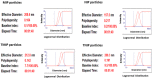
Bisphenol A (BPA) is an estrogen-mimicking chemical that can be selectively detected in water using a chemical sensor based on molecularly imprinted polymers (MIPs). However, the utility of BPA-MIPs in sensor applications is limited by the presence of non-specific binding sites. This study explored a dual approach to eliminating these sites: optimizing the molar ratio of the template (bisphenol A) to functional monomer (methacrylic acid) to cross-linker (ethylene glycol dimethacrylate), and esterifying the carboxylic acid residues outside of specific binding sites by treatment with diazomethane. The binding selectivity of treated MIPs and non-treated MIPs for BPA and several potential interferents was compared by capillary electrophoresis with ultraviolet detection. Baclofen, diclofenac and metformin were demonstrated to be good model interferents to test all MIPs for selective binding of BPA. Treated MIPs demonstrated a significant decrease in binding of the interferents while offering high selectivity toward BPA. These results demonstrate that conventional optimization of the molar ratio, together with advanced esterification of non-specific binding sites, effectively minimizes the residual binding of interferents with MIPs to facilitate BPA sensing.
Keywords: bisphenol A; diazomethane; non-specific binding sites; site-selective chemical modification; treated molecularly imprinted polymers.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
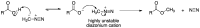

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

