Natural Products for the Treatment of Chlamydiaceae Infections
- PMID: 27754466
- PMCID: PMC5192522
- DOI: 10.3390/microorganisms4040039
Natural Products for the Treatment of Chlamydiaceae Infections
Abstract
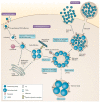
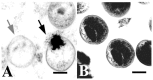
Due to the global prevalence of Chlamydiae, exploring studies of diverse antichlamydial compounds is important in the development of effective treatment strategies and global infectious disease management. Chlamydiaceae is the most widely known bacterial family of the Chlamydiae order. Among the species in the family Chlamydiaceae, Chlamydia trachomatis and Chlamydia pneumoniae cause common human diseases, while Chlamydia abortus, Chlamydia psittaci, and Chlamydia suis represent zoonotic threats or are endemic in human food sources. Although chlamydial infections are currently manageable in human populations, chlamydial infections in livestock are endemic and there is significant difficulty achieving effective treatment. To combat the spread of Chlamydiaceae in humans and other hosts, improved methods for treatment and prevention of infection are needed. There exist various studies exploring the potential of natural products for developing new antichlamydial treatment modalities. Polyphenolic compounds can inhibit chlamydial growth by membrane disruption, reestablishment of host cell apoptosis, or improving host immune system detection. Fatty acids, monoglycerides, and lipids can disrupt the cell membranes of infective chlamydial elementary bodies (EBs). Peptides can disrupt the cell membranes of chlamydial EBs, and transferrins can inhibit chlamydial EBs from attachment to and permeation through the membranes of host cells. Cellular metabolites and probiotic bacteria can inhibit chlamydial infection by modulating host immune responses and directly inhibiting chlamydial growth. Finally, early stage clinical trials indicate that polyherbal formulations can be effective in treating chlamydial infections. Herein, we review an important body of literature in the field of antichlamydial research.
Keywords: Chlamydia; Chlamydiaceae; Chlamydiae; antibacterial; chlamydial infections; natural products.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Chlamydial infections in wildlife-conservation threats and/or reservoirs of 'spill-over' infections?Vet Microbiol. 2016 Nov 30;196:78-84. doi: 10.1016/j.vetmic.2016.10.018. Epub 2016 Oct 15. Vet Microbiol. 2016. PMID: 27939160 Review.
-
Multiple Chlamydiaceae species in trachoma: implications for disease pathogenesis and control.PLoS Med. 2008 Jan 3;5(1):e14. doi: 10.1371/journal.pmed.0050014. PLoS Med. 2008. PMID: 18177205 Free PMC article.
-
Virulence-related comparative transcriptomics of infectious and non-infectious chlamydial particles.BMC Genomics. 2018 Aug 2;19(1):575. doi: 10.1186/s12864-018-4961-x. BMC Genomics. 2018. PMID: 30068313 Free PMC article.
-
Serotonin and melatonin, neurohormones for homeostasis, as novel inhibitors of infections by the intracellular parasite chlamydia.J Antimicrob Chemother. 2005 Nov;56(5):861-8. doi: 10.1093/jac/dki331. Epub 2005 Sep 19. J Antimicrob Chemother. 2005. PMID: 16172105
-
Chlamydiae from Down Under: The Curious Cases of Chlamydial Infections in Australia.Microorganisms. 2019 Nov 22;7(12):602. doi: 10.3390/microorganisms7120602. Microorganisms. 2019. PMID: 31766703 Free PMC article. Review.
Cited by
-
Effects of iota-carrageenan on ocular Chlamydia trachomatis infection in vitro and in vivo.J Appl Phycol. 2018;30(4):2601-2610. doi: 10.1007/s10811-018-1435-0. Epub 2018 Mar 13. J Appl Phycol. 2018. PMID: 30147240 Free PMC article.
-
Inhibitory Activity of Pyrroloisoxazolidine Derivatives against Chlamydia trachomatis.Biomed Res Int. 2021 Mar 13;2021:8889247. doi: 10.1155/2021/8889247. eCollection 2021. Biomed Res Int. 2021. PMID: 33791384 Free PMC article.
-
Resveratrol Inhibits Propagation of Chlamydia trachomatis in McCoy Cells.Biomed Res Int. 2017;2017:4064071. doi: 10.1155/2017/4064071. Epub 2017 Nov 29. Biomed Res Int. 2017. PMID: 29318147 Free PMC article.
-
Toxicity Assessment of Resveratrol Liposomes-in-Hydrogel Delivery System by EpiVaginalTM Tissue Model.Pharmaceutics. 2022 Jun 17;14(6):1295. doi: 10.3390/pharmaceutics14061295. Pharmaceutics. 2022. PMID: 35745867 Free PMC article.
-
Liposomes-In-Hydrogel Delivery System Enhances the Potential of Resveratrol in Combating Vaginal Chlamydia Infection.Pharmaceutics. 2020 Dec 11;12(12):1203. doi: 10.3390/pharmaceutics12121203. Pharmaceutics. 2020. PMID: 33322392 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

