Age-Dependent Metabolic and Immunosuppressive Effects of Tacrolimus
- PMID: 27754593
- PMCID: PMC5395364
- DOI: 10.1111/ajt.14087
Age-Dependent Metabolic and Immunosuppressive Effects of Tacrolimus
Abstract
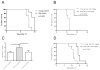
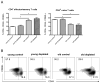
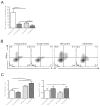
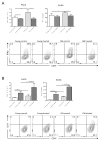
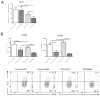
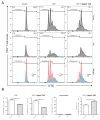
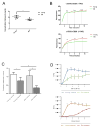
Immunosuppression in elderly recipients has been underappreciated in clinical trials. Here, we assessed age-specific effects of the calcineurin inhibitor tacrolimus (TAC) in a murine transplant model and assessed its clinical relevance on human T cells. Old recipient mice exhibited prolonged skin graft survival compared with young animals after TAC administration. More important, half of the TAC dose was sufficient in old mice to achieve comparable systemic trough levels. TAC administration was able to reduce proinflammatory interferon-γ cytokine production and promote interleukin-10 production in old CD4+ T cells. In addition, TAC administration decreased interleukin-2 secretion in old CD4+ T cells more effectively while inhibiting the proliferation of CD4+ T cells in old mice. Both TAC-treated murine and human CD4+ T cells demonstrated an age-specific suppression of intracellular calcineurin levels and Ca2+ influx, two critical pathways in T cell activation. Of note, depletion of CD8+ T cells did not alter allograft survival outcome in old TAC-treated mice, suggesting that TAC age-specific effects were mainly CD4+ T cell mediated. Collectively, our study demonstrates age-specific immunosuppressive capacities of TAC that are CD4+ T cell mediated. The suppression of calcineurin levels and Ca2+ influx in both old murine and human T cells emphasizes the clinical relevance of age-specific effects when using TAC.
Keywords: basic (laboratory) research/science; calcineurin inhibitor (CNI); graft survival; immunobiology; immunosuppressant; immunosuppression/immune modulation; translational research/science.
© 2016 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Figures

References
-
- World Population Ageing 2013. United Nations, Department of Economic and Social Affairs, Population Division; 2013. ST/ESA/SER.A/348.
-
- Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2012 Annual Data Report. U.S. Department of Health and Human Services, Health Resources and Services Administration; 2014.
-
- Karim A, Farrugia D, Cheshire J, Mahboob S, Begaj I, Ray D, et al. Recipient age and risk for mortality after kidney transplantation in England. Transplantation. 2014;97:832–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

