RhoA controls retinoid signaling by ROCK dependent regulation of retinol metabolism
- PMID: 27754752
- PMCID: PMC5997168
- DOI: 10.1080/21541248.2016.1248272
RhoA controls retinoid signaling by ROCK dependent regulation of retinol metabolism
Abstract
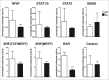
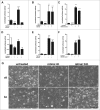
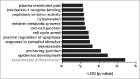
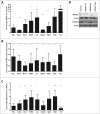
The ubiquitously expressed small GTPase RhoA is essential for embryonic development and mutated in different cancers. Functionally, it is well described as a regulator of the actin cytoskeleton, but its role in gene regulation is less understood. Using primary mouse keratinocytes with a deletion of the RhoA gene, we have now been exploring how the loss of RhoA affects gene expression. Performing transcription factor reporter assays, we found a significantly decreased activity of a RAR luciferase reporter in RhoA-null keratinocytes. Inhibition of the RhoA effector ROCK in control cells reproduced this phenotype. ATRA and retinal, but not retinol increased RAR reporter activity of keratinocytes with impaired RhoA/ROCK signaling, suggesting that retinol metabolism is regulated by RhoA/ROCK signaling. Furthermore a significant percentage of known ATRA target genes displayed altered expression in RhoA-null keratinocytes. These data reveal an unexpected link between the cytoskeletal regulator RhoA and retinoid signaling and uncover a novel pathway by which RhoA regulates gene expression.
Keywords: ROCK; RhoA; retinoic acid; skin; stem cells.
Figures



Similar articles
-
Blocking RhoA/ROCK inhibits the pathogenesis of pemphigus vulgaris by suppressing oxidative stress and apoptosis through TAK1/NOD2-mediated NF-κB pathway.Mol Cell Biochem. 2017 Dec;436(1-2):151-158. doi: 10.1007/s11010-017-3086-x. Epub 2017 Jun 12. Mol Cell Biochem. 2017. PMID: 28608226
-
RhoA is dispensable for skin development, but crucial for contraction and directed migration of keratinocytes.Mol Biol Cell. 2011 Mar 1;22(5):593-605. doi: 10.1091/mbc.E09-10-0859. Epub 2011 Jan 5. Mol Biol Cell. 2011. PMID: 21209320 Free PMC article.
-
Ras homolog family member A/Rho-associated protein kinase 1 signaling modulates lineage commitment of mesenchymal stem cells in asthmatic patients through lymphoid enhancer-binding factor 1.J Allergy Clin Immunol. 2019 Apr;143(4):1560-1574.e6. doi: 10.1016/j.jaci.2018.08.023. Epub 2018 Sep 5. J Allergy Clin Immunol. 2019. PMID: 30194990 Free PMC article.
-
Novel cardiovascular protective effects of RhoA signaling and its therapeutic implications.Biochem Pharmacol. 2023 Dec;218:115899. doi: 10.1016/j.bcp.2023.115899. Epub 2023 Oct 30. Biochem Pharmacol. 2023. PMID: 37907138 Review.
-
RhoA as a Key Regulator of Innate and Adaptive Immunity.Cells. 2019 Jul 17;8(7):733. doi: 10.3390/cells8070733. Cells. 2019. PMID: 31319592 Free PMC article. Review.
Cited by
-
Role of cellular retinol-binding protein, type 1 and retinoid homeostasis in the adult mouse heart: A multi-omic approach.FASEB J. 2022 Apr;36(4):e22242. doi: 10.1096/fj.202100901RRR. FASEB J. 2022. PMID: 35253263 Free PMC article.
-
The versatility of RhoA activities in neural differentiation.Small GTPases. 2019 Jan;10(1):26-32. doi: 10.1080/21541248.2016.1273171. Epub 2017 Jan 27. Small GTPases. 2019. PMID: 28125332 Free PMC article.
-
MIRO2 promotes cancer invasion and metastasis via MYO9B suppression of RhoA activity.Cell Rep. 2025 Jan 28;44(1):115120. doi: 10.1016/j.celrep.2024.115120. Epub 2024 Dec 24. Cell Rep. 2025. PMID: 39723893 Free PMC article.
-
Retinoic acid signaling promotes the cytoskeletal rearrangement of embryonic epicardial cells.FASEB J. 2018 Jul;32(7):3765-3781. doi: 10.1096/fj.201701038R. Epub 2018 Feb 15. FASEB J. 2018. PMID: 29447006 Free PMC article.
-
An 8‑gene signature predicts the prognosis of cervical cancer following radiotherapy.Mol Med Rep. 2019 Oct;20(4):2990-3002. doi: 10.3892/mmr.2019.10535. Epub 2019 Jul 29. Mol Med Rep. 2019. PMID: 31432147 Free PMC article.
References
-
- Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Bi 2005; 21:247-69; https://doi.org/10.1146/annurev.cellbio.21.020604.150721 - DOI - PubMed
-
- Pedersen E, Brakebusch C. Rho GTPase function in development: how in vivo models change our view. Exp Cell Res 2012; 318:1779-87; PMID:22659168; https://doi.org/10.1016/j.yexcr.2012.05.004 - DOI - PubMed
-
- Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. BioEssays 2007; 29:356-70; PMID:17373658; https://doi.org/10.1002/bies.20558 - DOI - PMC - PubMed
-
- Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, Meyerson M, Gabriel SB, Lander ES, Getz G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014; 505:495-501; PMID:24390350; https://doi.org/10.1038/nature12912 - DOI - PMC - PubMed
-
- Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, Siu HC, Deng S, Chu KM, Law S, et al.. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet 2014; 46:573-82; PMID:24816253; https://doi.org/10.1038/ng.2983 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
