Hepatic Glucocorticoid Receptor Plays a Greater Role Than Adipose GR in Metabolic Syndrome Despite Renal Compensation
- PMID: 27754788
- PMCID: PMC5133352
- DOI: 10.1210/en.2016-1615
Hepatic Glucocorticoid Receptor Plays a Greater Role Than Adipose GR in Metabolic Syndrome Despite Renal Compensation
Abstract
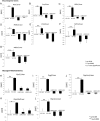
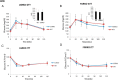
Exogenous glucocorticoid administration results in hyperglycemia, insulin resistance, hepatic dyslipidemia, and hypertension, a constellation of findings known as Cushing's syndrome. These effects are mediated by the glucocorticoid receptor (GR). Because GR activation in liver and adipose has been implicated in metabolic syndrome (MS), we wanted to determine the role of GR in these tissues in the development of MS. Because GR knockout (KO) mice (whole-body KO) exhibit perinatal lethality due to respiratory failure, we generated tissue-specific (liver or adipose) GRKO mice using cre-lox technology. Real-time PCR analysis of liver mRNA from dexamethasone-treated wildtype (WT) and liver GRKO mice indicated that hepatic GR regulates the expression of key genes involved in gluconeogenesis and glycogen metabolism. Interestingly, we have observed that liver-specific deletion of GR resulted in a significant increase in mRNA expression of key genes involved in gluconeogenesis and glycogen metabolism in kidney tissue, indicating a compensatory mechanism to maintain glucose homeostasis. We have also observed that GR plays an important role in regulating the mRNA expression of key genes involved in lipid metabolism. Liver GRKO mice demonstrated decreased fat mass and liver glycogen content compared with WT mice administered dexamethasone for 2 weeks. Adipose-specific deletion of GR did not alter glucose tolerance or insulin sensitivity of adipose GRKO mice compared with WT mice administrated dexamethasone. This indicates that liver GR might be more important in development of MS in dexamethasone-treated mice, whereas adipose GR plays a little role in these paradigms.
Figures






References
-
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. - PubMed
-
- Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. - PubMed
-
- Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10(2):213–219. - PubMed
-
- Greenberg AK, Hu J, Basu S, et al. Glucocorticoids inhibit lung cancer cell growth through both the extracellular signal-related kinase pathway and cell cycle regulators. Am J Respir Cell Mol Biol. 2002;27(3):320–328. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

