A Model of Differential Growth-Guided Apical Hook Formation in Plants
- PMID: 27754878
- PMCID: PMC5134968
- DOI: 10.1105/tpc.15.00569
A Model of Differential Growth-Guided Apical Hook Formation in Plants
Abstract
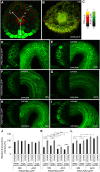
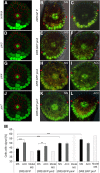
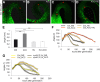
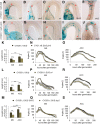
Differential cell growth enables flexible organ bending in the presence of environmental signals such as light or gravity. A prominent example of the developmental processes based on differential cell growth is the formation of the apical hook that protects the fragile shoot apical meristem when it breaks through the soil during germination. Here, we combined in silico and in vivo approaches to identify a minimal mechanism producing auxin gradient-guided differential growth during the establishment of the apical hook in the model plant Arabidopsis thaliana Computer simulation models based on experimental data demonstrate that asymmetric expression of the PIN-FORMED auxin efflux carrier at the concave (inner) versus convex (outer) side of the hook suffices to establish an auxin maximum in the epidermis at the concave side of the apical hook. Furthermore, we propose a mechanism that translates this maximum into differential growth, and thus curvature, of the apical hook. Through a combination of experimental and in silico computational approaches, we have identified the individual contributions of differential cell elongation and proliferation to defining the apical hook and reveal the role of auxin-ethylene crosstalk in balancing these two processes.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures


References
-
- Blilou I., Xu J., Wildwater M., Willemsen V., Paponov I., Friml J., Heidstra R., Aida M., Palme K., Scheres B. (2005). The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

