Open-label placebo treatment in chronic low back pain: a randomized controlled trial
- PMID: 27755279
- PMCID: PMC5113234
- DOI: 10.1097/j.pain.0000000000000700
Open-label placebo treatment in chronic low back pain: a randomized controlled trial
Erratum in
-
Erratum.Pain. 2017 Feb;158(2):365. doi: 10.1097/j.pain.0000000000000820. Pain. 2017. PMID: 28092652 Free PMC article. No abstract available.
Abstract
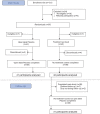
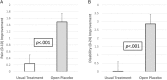
This randomized controlled trial was performed to investigate whether placebo effects in chronic low back pain could be harnessed ethically by adding open-label placebo (OLP) treatment to treatment as usual (TAU) for 3 weeks. Pain severity was assessed on three 0- to 10-point Numeric Rating Scales, scoring maximum pain, minimum pain, and usual pain, and a composite, primary outcome, total pain score. Our other primary outcome was back-related dysfunction, assessed on the Roland-Morris Disability Questionnaire. In an exploratory follow-up, participants on TAU received placebo pills for 3 additional weeks. We randomized 97 adults reporting persistent low back pain for more than 3 months' duration and diagnosed by a board-certified pain specialist. Eighty-three adults completed the trial. Compared to TAU, OLP elicited greater pain reduction on each of the three 0- to 10-point Numeric Rating Scales and on the 0- to 10-point composite pain scale (P < 0.001), with moderate to large effect sizes. Pain reduction on the composite Numeric Rating Scales was 1.5 (95% confidence interval: 1.0-2.0) in the OLP group and 0.2 (-0.3 to 0.8) in the TAU group. Open-label placebo treatment also reduced disability compared to TAU (P < 0.001), with a large effect size. Improvement in disability scores was 2.9 (1.7-4.0) in the OLP group and 0.0 (-1.1 to 1.2) in the TAU group. After being switched to OLP, the TAU group showed significant reductions in both pain (1.5, 0.8-2.3) and disability (3.4, 2.2-4.5). Our findings suggest that OLP pills presented in a positive context may be helpful in chronic low back pain.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures
Comment in
-
Are placebo pills presented as experimental treatment a true placebo?Pain. 2017 Mar;158(3):535. doi: 10.1097/j.pain.0000000000000793. Pain. 2017. PMID: 28187103 No abstract available.
-
Placebo and chronic low back pain: too much in way of expectations, too little in terms of data.Pain. 2017 Mar;158(3):535-536. doi: 10.1097/j.pain.0000000000000794. Pain. 2017. PMID: 28187104 No abstract available.
-
The effect of nothing? Time to abandon the concept of placebo.Pain. 2017 Jun;158(6):1179. doi: 10.1097/j.pain.0000000000000884. Pain. 2017. PMID: 28514257 No abstract available.
-
Placebo pills provided without deception may help to reduce pain and disability in people with chronic low back pain [commentary].J Physiother. 2017 Jul;63(3):183. doi: 10.1016/j.jphys.2017.05.001. Epub 2017 Jun 17. J Physiother. 2017. PMID: 28633881 No abstract available.
-
Placebo pills provided without deception may help to reduce pain and disability in people with chronic low back pain [synopsis].J Physiother. 2017 Jul;63(3):183. doi: 10.1016/j.jphys.2017.05.002. Epub 2017 Jun 20. J Physiother. 2017. PMID: 28641895 No abstract available.
References
-
- Andersson GB. Epidemiological features of chronic low-back pain. Lancet 1999;354:581–5. - PubMed
-
- Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S, Turk DC. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane Review. Spine 2014;39:556–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

