Hypersensitivity Induced by Activation of Spinal Cord PAR2 Receptors Is Partially Mediated by TRPV1 Receptors
- PMID: 27755539
- PMCID: PMC5068818
- DOI: 10.1371/journal.pone.0163991
Hypersensitivity Induced by Activation of Spinal Cord PAR2 Receptors Is Partially Mediated by TRPV1 Receptors
Abstract
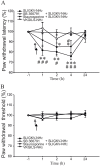
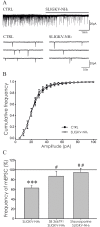
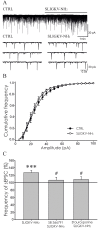
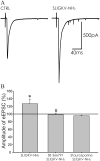
Protease-activated receptors 2 (PAR2) and transient receptor potential vanilloid 1 (TRPV1) receptors in the peripheral nerve endings are implicated in the development of increased sensitivity to mechanical and thermal stimuli, especially during inflammatory states. Both PAR2 and TRPV1 receptors are co-expressed in nociceptive dorsal root ganglion (DRG) neurons on their peripheral endings and also on presynaptic endings in the spinal cord dorsal horn. However, the modulation of nociceptive synaptic transmission in the superficial dorsal horn after activation of PAR2 and their functional coupling with TRPV1 is not clear. To investigate the role of spinal PAR2 activation on nociceptive modulation, intrathecal drug application was used in behavioural experiments and patch-clamp recordings of spontaneous, miniature and dorsal root stimulation-evoked excitatory postsynaptic currents (sEPSCs, mEPSCs, eEPSCs) were performed on superficial dorsal horn neurons in acute rat spinal cord slices. Intrathecal application of PAR2 activating peptide SLIGKV-NH2 induced thermal hyperalgesia, which was prevented by pretreatment with TRPV1 antagonist SB 366791 and was reduced by protein kinases inhibitor staurosporine. Patch-clamp experiments revealed robust decrease of mEPSC frequency (62.8 ± 4.9%), increase of sEPSC frequency (127.0 ± 5.9%) and eEPSC amplitude (126.9 ± 12.0%) in dorsal horn neurons after acute SLIGKV-NH2 application. All these EPSC changes, induced by PAR2 activation, were prevented by SB 366791 and staurosporine pretreatment. Our results demonstrate an important role of spinal PAR2 receptors in modulation of nociceptive transmission in the spinal cord dorsal horn at least partially mediated by activation of presynaptic TRPV1 receptors. The functional coupling between the PAR2 and TRPV1 receptors on the central branches of DRG neurons may be important especially during different pathological states when it may enhance pain perception.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Spinal PAR2 Activation Contributes to Hypersensitivity Induced by Peripheral Inflammation in Rats.Int J Mol Sci. 2021 Jan 20;22(3):991. doi: 10.3390/ijms22030991. Int J Mol Sci. 2021. PMID: 33498178 Free PMC article.
-
TRPV1 receptor inhibition decreases CCL2-induced hyperalgesia.Neuropharmacology. 2014 Jun;81:75-84. doi: 10.1016/j.neuropharm.2014.01.041. Epub 2014 Feb 1. Neuropharmacology. 2014. PMID: 24495396
-
The role of the TRPV1 endogenous agonist N-Oleoyldopamine in modulation of nociceptive signaling at the spinal cord level.J Neurophysiol. 2009 Jul;102(1):234-43. doi: 10.1152/jn.00024.2009. Epub 2009 Apr 15. J Neurophysiol. 2009. PMID: 19369364
-
The role of spinal cord vanilloid (TRPV1) receptors in pain modulation.Physiol Res. 2008;57 Suppl 3:S69-S77. doi: 10.33549/physiolres.931601. Epub 2008 May 13. Physiol Res. 2008. PMID: 18481913 Review.
-
Anandamide-Mediated Modulation of Nociceptive Transmission at the Spinal Cord Level.Physiol Res. 2024 Aug 30;73(S1):S435-S448. doi: 10.33549/physiolres.935371. Epub 2024 Jul 2. Physiol Res. 2024. PMID: 38957948 Free PMC article. Review.
Cited by
-
Transient receptor potential vanilloid subtype 1 depletion mediates mechanical allodynia through cellular signal alterations in small-fiber neuropathy.Pain Rep. 2021 Apr 2;6(1):e922. doi: 10.1097/PR9.0000000000000922. eCollection 2021. Pain Rep. 2021. PMID: 34585035 Free PMC article. Review.
-
Social disruption-induced stress pre-exposure aggravates, while the presence of conspecifics diminishes, acetic acid-induced writhing.Psychopharmacology (Berl). 2021 Oct;238(10):2851-2865. doi: 10.1007/s00213-021-05901-z. Epub 2021 Jun 28. Psychopharmacology (Berl). 2021. PMID: 34181036
-
Cathepsin S acts via protease-activated receptor 2 to activate sensory neurons and induce itch-like behaviour.Neurobiol Pain. 2019 May 2;6:100032. doi: 10.1016/j.ynpai.2019.100032. eCollection 2019 Aug-Dec. Neurobiol Pain. 2019. PMID: 31223140 Free PMC article.
-
TRPV1 Channel Activated by the PGE2/EP4 Pathway Mediates Spinal Hypersensitivity in a Mouse Model of Vertebral Endplate Degeneration.Oxid Med Cell Longev. 2021 Aug 21;2021:9965737. doi: 10.1155/2021/9965737. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34471470 Free PMC article.
-
N-Acetylcysteine causes analgesia in a mouse model of painful diabetic neuropathy.Mol Pain. 2020 Jan-Dec;16:1744806920904292. doi: 10.1177/1744806920904292. Mol Pain. 2020. PMID: 32009537 Free PMC article.
References
-
- Vergnolle N (2000) Review article: proteinase-activated receptors—novel signals for gastrointestinal pathophysiology. Aliment Pharmacol Ther 14: 257–266. - PubMed
-
- Nystedt S, Emilsson K, Larsson AK, Strombeck B, Sundelin J (1995) Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur J Biochem 232: 84–89. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

